Fig. 4. LiZIP3 is a cell surface zinc transporter in L. infantum.
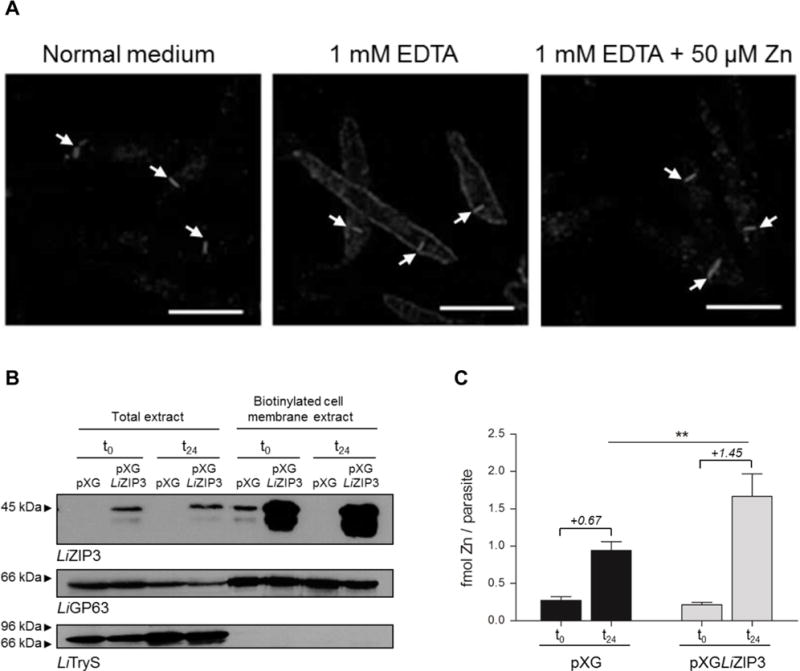
A. Zinc restriction stimulates the expression of LiZIP3 at the cell membrane of L. infantum. Parasites were grown at an initial density of 106 mL−1 in normal medium or in the presence of 1 mM EDTA. At 48 h of growth, 50 μM zinc were added to an aliquot of the EDTA-treated culture and, 24 h later, LiZIP3 localization was assessed by immunofluorescence using the anti-LiZIP3 antibody. The position of the kinetoplast in each parasite, observed by simultaneously staining samples with DAPI, is indicated by arrows. Scale bar: 5 μm. B and C. Overexpression of LiZIP3 at the cell surface results in an increased uptake of zinc.
B. Control (pXG) and LiZIP3-overexpressing (pXGLIZIP3) parasites with 72 h growth were exposed to a zinc stimulus by dilution to 106 mL−1 in fresh medium and cultured for an additional 24 h. The presence of LiZIP3 at the cell membrane, before (t0) and 24 h after (t24) medium replacement, was analysed by western blot analysis of cell membrane biotinylated proteins (the faster migrating band observed at approximately 38 kDa refers to a protein of unknown identity that is often recognized by the anti-LiZIP3 antibody). Upon stripping, LiGP63 and trypanothione synthetase (LiTryS) (Sousa et al., 2014) were used as controls for cell membrane and cytosolic proteins, respectively.
C. The zinc content of pXG and LiZIP3-overexpressing parasites was measured by atomic absorption spectrometry at t0 and t24 (mean ± SD, n = 3; asterisks indicate significant differences between pXG and LiZIP3-overexpressing parasites **P < 0.01, Student’s t test). Numbers in italic refer to the mean of the differences between zinc content at t0 and t24 for each parasite line.
