Fig. 7. Expression of LiZIP3 is regulated by zinc at the mRNA stability level through a process involving a short-lived protein.
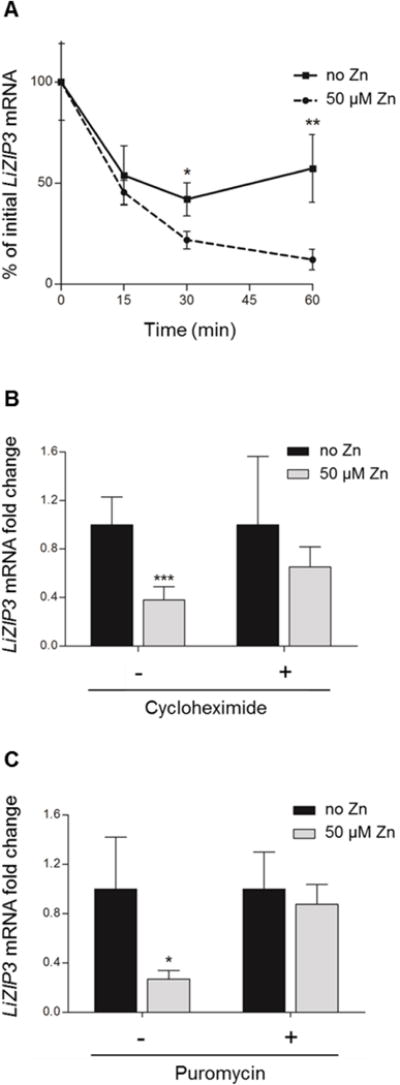
A. Effect of zinc on the degradation of LiZIP3 mRNA. Messenger RNA synthesis was blocked with 10 μg mL−1 actinomycin D and the degradation of LiZIP3 mRNA in the absence and in the presence of 50 μM zinc was accompanied by qRT-PCR (mean ± SD, n = 3–4; asterisks indicate significant differences in each time-point, *P < 0.05, **P < 0.01, Student’s t test).
B and C. Down-regulation of LiZIP3 mRNA requires the activity of a labile protein. Promastigotes grown for 48 h in normal medium were incubated with 50 μg mL−1 cycloheximide (B) or 200 μg mL−1 puromycin (C) during 2 h prior to addition of 50 μM zinc for 1 h. Fold changes in LiZIP3 mRNA were measured by qRT-PCR (mean ± SD, n = 5 for cycloheximide, n = 3 for puromycin; asterisks indicate significant differences between cultures not supplemented and supplemented with zinc in each condition tested, i.e. with or without cycloheximide or puromycin, *P < 0.05, ***P < 0.001, Student’s t test).
