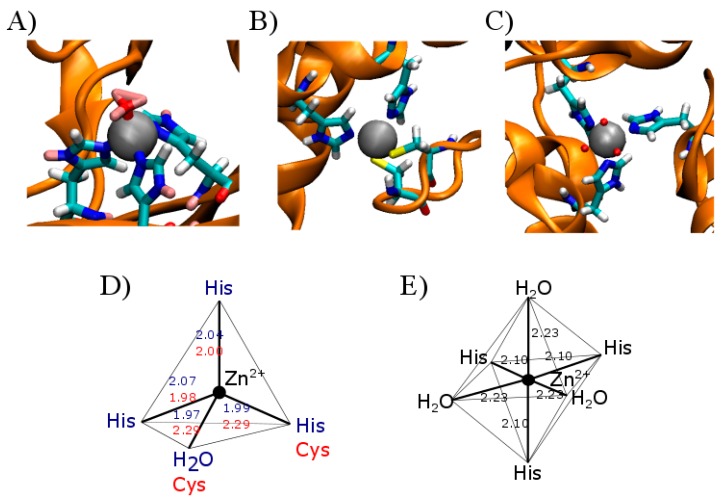
Figure 1.
Representative examples of zinc-containing proteins and the representation of their corresponding metal coordination geometries. The illustration shows a representation of the zinc binding pockets of two classic metalloproteins. (A) shows the zinc binding motif in carbonic anhydrase II (PDB: 3KKX) and its corresponding coordination sphere (D) in blue, including the length of the coordination bonds in angstroms; (B) represents a zinc finger motif present in the human enhancer binding protein MBP-1 (PDB:1BBO) with its corresponding coordination sphere (D) in red, including binding lengths; (C) represents hexameric human insulin (PDB: 1MCO) with its Zn coordinating sphere (E) and the corresponding atom binding lengths. The light blue ball in (A–C) represents the zinc atom; dark blue represents the imidazole nitrogen involved in the coordination sphere, while red represents the oxygen, and yellow the sulfur atoms interacting with zinc. The interactions are shown by the dashed line in light blue. In (D,E) the atomic distances between zinc and its coordination sphere are indicated in angstroms. Blue numbers in (D) apply to (A), red in (D) apply to (B) and black in (E) correspond to (C) as detailed.