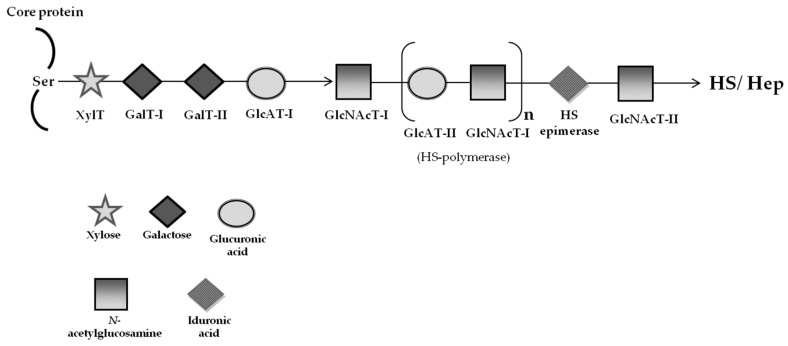
Figure 2.
Schematic presentation of the biosynthetic assembly of heparan sulfate (HS) and heparin (Hep) from GAG backbones through the action of several glycosyltransferases (adapted from [106]). Each glycosyltransferase requires the respective UDP-sugar as a donor substrate. Following the synthesis of specific core proteins, the synthesis of the so-called GAG-protein linkage region, GlcUA β1—3Gal β1—3Gal β1—4Xylβ1-O-, common to chondroitin sulfate/dermatan sulfate (CS/DS) and HS/Hep chains, is initiated by XylT, which transfers a Xyl residue from UDP-Xyl to the specific Ser residue in the endoplasmic reticulum, and is completed by the consecutive addition of each sugar by GalT-I, GalT-II, and GlcAT-I, which are common to the biosynthesis of both CS and HS, in the Golgi apparatus. The addition of 1–4-linked GlcNAc to the linkage region by GlcNAcT-I initiates the assembly of the HS repeating disaccharide region, (-4GlcNAcβ1—4GlcUAβ1-)n. Then, the chain polymerization of the HS chain is catalyzed by HS-GlcAT-II and GlcNAcT-II activities of HS polymerase, which is a heterocomplex of EXT1 and EXT2. After the formation of the heparan backbone, GAG chains are matured by sulfation at various positions and epimerization at GlcUA residues. Each enzyme (glycosyltransferase and/or epimerase) is described by its respective sugar symbol: β-xylosyltransferase (XylT); β-1,4-galactosyltransferase-I (GalT-I); β-1,3-galactosyltransferase-II (GalT-II); β-1,3-glucuronosyltransferases (GlcAT-I and GlcAT-II); and 1,4-N-acetylglucosaminyltransferase (GlcNAcT-I). Sulfotransferases involved in the chain modifications are not included.