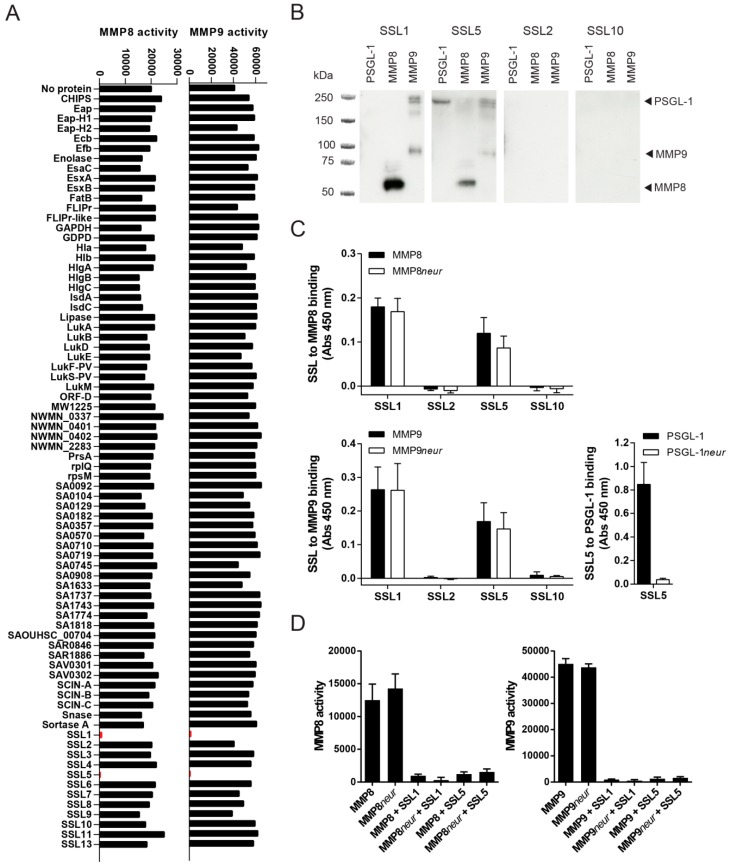
Figure 1.
Identification of SSL1 and SSL5 as inhibitors of neutrophil MMPs. (A) The effects of 76 secreted staphylococcal proteins (10 µg/mL) on the activity of MMP8 (left) and MMP9 (right) was assessed by measuring the conversion of a fluorogenic peptide substrate. SSL1 and SSL5 are shown in red. One representative out of three separate experiments is shown; (B) SSL to MMP binding was visualized in far Western blot. PSGL-1 (lane 1), MMP8 (lane 2), MMP9 (lane 3), each 0.2 µg/lane, were loaded on gel and subsequently blotted and incubated with 10 µg/mL HIS-tagged SSLs and detected with anti-HIS (anti-X-Press). The expected height of all proteins is indicated on the right. One representative experiment is shown; (C) Neuraminidase treatment (neur) of 3 µg/mL coated MMP8 (upper graph) and MMP9 (lower graph) did not alter SSL to MMP binding, as measured by anti-HIS detection in ELISA, whereas it did abrogate SSL5 to PSGL-1 (coated, 3 µg/mL) binding (right graph); Data points represent the mean and standard error (SE) from three independent experiments; (D) Neur treatment of MMP8 (left graph) and MMP9 (right graph) did not alter the inhibitory activity of 10 µg/mL SSL1 and SSL5 on MMP8/9 activity as measured in the fluorogenic peptide substrate assay. Data points represent the mean fluorescence and SE of three independent experiments.