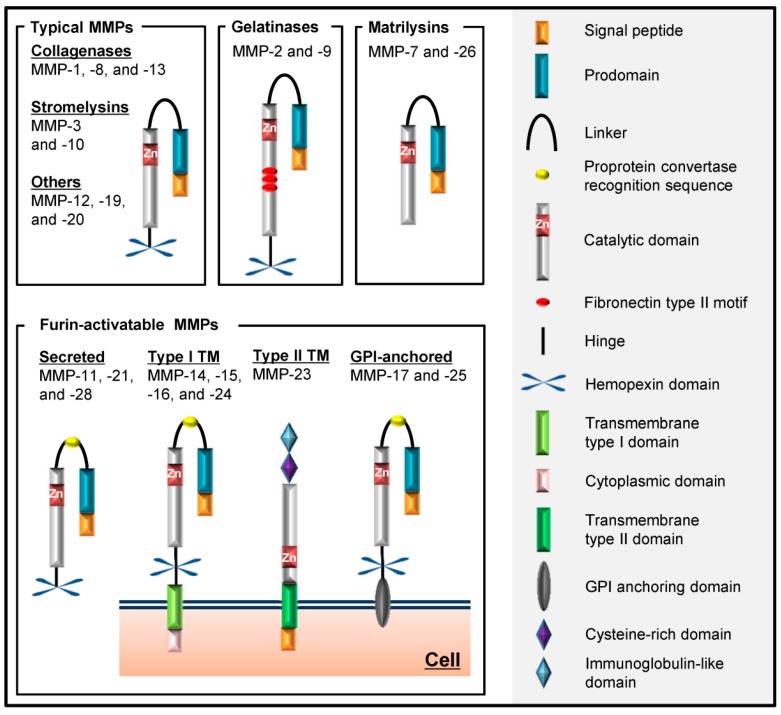
Figure 2.
The schematic structures of the MMP family. Matrix metalloproteinases (MMPs) have four basic domains: the signal peptide responsible for secretion, the prodomain, which keeps the MMP inactive by coordinating the zinc ion of the catalytic site, the catalytic domain responsible for the proteolytic activity and the hemopexin domain of a propeller blade structure. The gelatinases contain three fibronectin type II repeats, which bind gelatin. Matrilysins and MMP-23 lack the hinge region and the hemopexin domain. The membrane-type MMPs (MT-MMPs) are localized on the cell surface anchored by a transmembrane (TM) domain or a glycosylphosphatidyl-inositol (GPI) anchor.