Table 3.
Estimated Marginal (EM) and Observed Means of Standardized Neurocognitive Domain Scores for Groups With Attention-Deficit/Hyperactivity Disorder (ADHD) Relative to the Non-Clinical Comparison Group’s Baseline Scores for Each Treatment Condition
| Working Memory | Inhibition | RTV | RT | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Base- line |
Week 4 | Week 8 | Base- line |
Week 4 | Week 8 |
Base- line |
Week 4 |
Week 8 | Base- line |
Week 4 | Week 8 |
|
| GUAN | ||||||||||||
| Med | NONE | GUAN | GUAN | NONE | GUAN | GUAN | NONE | GUAN | GUAN | NONE | GUAN | GUAN |
| EM | −.53 (.04) |
−.53 (.05) |
−.60 (.06) |
−.22 (.04) |
−.03 (.06) |
.11 (.07) |
−.32 (.05) |
−.11 (.07) |
.04 (.08) |
−.14 (.05) |
.06 (.06) |
.22 (.07) |
| Observed | −.48 (.08) |
−.52 (.13) |
−.36 (.10) |
−.13 (.06) |
.08 (.16) |
.08 (.07) |
−.31 (.11) |
.03 (.16) |
−.15 (.10) |
−.09 (.08) |
.09 (.12) |
.14 (.10) |
| COMB | ||||||||||||
| Med | NONE | GUAN | COMB | NONE | GUAN | COMB | NONE | GUAN | COMB | NONE | GUAN | COMB |
| EM | −.53 (.04) |
−.53 (.05) |
−.25 (.07) |
−.22 (.04) |
−.03 (.06) |
.15 (.09) |
−.32 (.05) |
−.11 (.07) |
.24 (.09) |
−.14 (.05) |
.06 (.06) |
.13 (.09) |
| Observed | −.48 (.08) |
−.58 (.13) |
−.25 (.10) |
−.28 (.08) |
−.07 (.15) |
.13 (.07) |
−.50 (.10) |
−.06 (.15) |
.17 (.11) |
−.23 (.08) |
.16 (.14) |
.13 (.11) |
| DMPH | ||||||||||||
| Med | NONE | NONE | DMPH | NONE | NONE | DMPH | NONE | NONE | DMPH | NONE | NONE | DMPH |
| EM | −.53 (.04) |
−.60 (.06) |
−.30 (.07) |
−.22 (.04) |
−.09 (.07) |
.08 (.07) |
−.32 (.05) |
−.17 (.08) |
.18 (.09) |
−.14 (.05) |
.01 (.07) |
.26 (.09) |
| Observed | −.57 (.07) |
−.79 (.10) |
−.38 (.07) |
−.33 (.08) |
.01 (.14) |
.07 (.05) |
−.31 (.08) |
.13 (.14) |
.25 (.08) |
−.22 (.10) |
.13 (.13) |
.28 (.09) |
Note: Scores are coded so that higher values reflect better performance (negative values represent impairments relative to the comparison group; for response time (RT) and RT variability (RTV), lower scores were considered better). The shading in each cell indicates the treatments received at each time-point (white = no treatment or placebo, 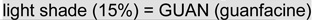 ,
,  d-methylphenidate),
d-methylphenidate),  ): at baseline none of the groups had yet received treatment; at week 4 both GUAN and combination (COMB) groups were receiving guanfacine, while the DMPH group remained on placebo; and at week 8 the GUAN group was receiving guanfacine, the COMB group was receiving the combined treatment, and the DMPH group was receiving only DMPH. Note that the placebo effect is estimated based on performance observed across the 3 groups at baseline and in the DMPH group at week 4.
): at baseline none of the groups had yet received treatment; at week 4 both GUAN and combination (COMB) groups were receiving guanfacine, while the DMPH group remained on placebo; and at week 8 the GUAN group was receiving guanfacine, the COMB group was receiving the combined treatment, and the DMPH group was receiving only DMPH. Note that the placebo effect is estimated based on performance observed across the 3 groups at baseline and in the DMPH group at week 4.
