ABSTRACT
It is strategically important to monitor the safety profile of vaccination schedules in order to achieve and maintain high levels of coverage. We analyzed the cohort of individuals actively invited for measles, mumps, rubella and varicella (MMRV) vaccination in the Veneto region (north-east Italy) from 8/1/2013 to 7/31/2014, assessing the onset of adverse events (AE) relating to 2 different vaccination strategies for MMRV (MMR+V vs MMRV).
During the vaccination session at 14 months old, parents were given a form for recording local and systemic reactions to vaccinations for 4 weeks afterwards. Overall, 12,288 forms were returned, and 84.6% of them were included in this analysis (5,130 relating to MMR+V and 5,265 to MMRV); 37.3% of the sample reported no AEs, with no difference between the 2 groups. Local reactions were more common in the MMR+V group (9.6% vs 2.9%; RR 3.33; 95% CI 2.79–3.98), while there was no difference in general reactions between the 2 groups (50% MMR+V vs 52% MMRV). The events most often reported were “fever <39.5°C,” which was more frequently associated with the MMRV strategy (p<0.001), and “skin blotches and marks,” which occurred more often in the MMR+V group (p<0.001). Reports of “fever ≥39.5°C” were equally distributed between the 2 groups. Sixteen cases of febrile seizures were reported (0.14% in the MMR+V group and 0.17% in the MMRV group).
Similar safety profiles were identified for the 2 vaccination strategies. Although the method used to record reactions to vaccination demanded considerable resources, it enabled important information to be collected on parents' perception of the AEs occurring in response to their child's vaccination.
Keywords: active surveillance, adverse event, febrile seizure, MMRV, MMR+V
Attenuated viral vaccines against measles, mumps, rubella and varicella have been associated with several adverse events, including fever and febrile seizures.1-5 After the combined vaccine was licensed, the Advisory Committee on Immunization Practices stated that the administration of separate measles, mumps, rubella (MMR) and varicella (V) vaccines on the same day was preferred for the first recommended dose at 12 to 15 months of age (MMR+V).6,7
In 2005, as part of the Veneto Region's routine childhood immunization program, the chickenpox vaccine was offered to children at 14 months of age, in co-administration with MMR, with a second dose of varicella vaccine for 6-year-old children and a catch-up dose for 12 year-olds with a negative history of varicella (Regional Law No. 4403 of 30 December 2004).8
For the first year of the vaccination program, the target was to achieve a 60% adherence rate, given that the program began with 2 separate MMR and varicella vaccines (the combined MMRV vaccine became available in 2007); the adherence rate was actually over 80%.9 New cases of varicella dropped from about 61,000 in 2004 to 23,600 in 2008 and the hospitalization rates decreased from 18.7/100,000 in 2000 to 0.8/100,000 in 2012.10
For combined MMRV vaccines, the most salient safety issue emerging after their widespread use in routine practice was a higher risk of febrile seizures. Analyses on post-marketing studies conducted on children receiving their first dose of MMRV vaccine have shown that they had febrile seizures more frequently than children vaccinated with separate varicella and MMR vaccines.11-13 As a result, the Veneto Regional Authority's recommendations left up to each local public health unit to decide which strategy to use in relation to their local epidemiological context.14
In Italy the reporting of adverse events following immunizations (AEFIs) is regulated by a Ministerial Decree issued in 2003.15 and in the Veneto Region the Green Channel created by the local public health authority is integrated with the national surveillance system to offer advice on vaccinations at risk of adverse events and to ensure an efficient AEFI surveillance system with regular feedback for vaccination personnel. 16
The present study analyses the adverse events (AEs) recorded by means of an active surveillance comparing 2 alternative vaccination schedules (MMRV and MMR+V) in a sample of children in the Veneto Region, particularly focusing on febrile seizures.
This observational prospective population-based cohort study was conducted at 6 public health units in the Veneto Region between 1st August 2013 and 31st July 2014. Written informed consent was obtained from all children's parents or guardians before their inclusion in the study.
Children were eligible for the study if they were healthy at the time of their first vaccination against measles, mumps, rubella and varicella. We included subjects given the combined MMRV vaccine (Priorix-Tetra®, GlaxoSmithKline) or separate MMR (Priorix®, GlaxoSmithKline or MMRVAXPRO®, Sanofi Pasteur MSD) and varicella (Varivax®, Sanofi Pasteur MSD or Varilrix®, GlaxoSmithKline) vaccines administered on the same day as part of the routine childhood immunization program.
At the time of their children's vaccination, parents/guardians were given a form and instructions on how to record local and general symptoms solicited by the vaccinations, as well as any other reactions. The first section was completed by the health service operator administering the vaccine, and contained personal details and information on the type of vaccine administered, the date of vaccination, the vaccine batch, and the site and side of inoculation. The second section was for parents to record any common reactions, distinguishing between local (pain, swelling, redness, blisters) and systemic events (fever, irritability, restlessness, drowsiness, blotches or marks on the skin, swelling of the cheeks or ear, swollen lymph glands in the neck, afebrile and febrile seizures, and so on), and specifying the date of onset and duration. Parents were also asked to record any allergic reactions occurring within 2 hours from the inoculation (urticaria or others), any use of medication or resort to health care facilities, medical examinations or laboratory tests. The forms were returned to the public health unit a month later, at the child's next vaccination appointment.
A standardized clinical and causality assessment framework was applied to classify cases as definite, probable, possible, unlikely, unrelated, or unclassifiable based on the data recorded on the form.17 Subjects were kept under observation for 15 minutes after the vaccines were injected to check for any immediate reactions, as confirmed and recorded by healthcare providers. Local reactions such as ecchymosis, erythema, induration, swelling and pain at the injection site, and systemic reactions such as arthralgia, chills, fever, headache, malaise, myalgia, fatigue and sweating were recorded on each subject's form for 7 d after vaccination. Then any moderate or serious AEs occurring during the subsequent 4 weeks after vaccination were recorded. During this observational period, any additional vaccination was performed in accordance with the Veneto's vaccination schedule.
AEs rates were calculated per 100 vaccinations. Data were analyzed using Epi Info ™ 7.1.4.0 (Centers for Disease Control and Prevention (CDC) Atlanta). The chi-square test and Student's t-test were conducted, and relative risks (RR) with binomial 95% confidence intervals (CI) were calculated to analyze any differences between groups of subjects, as appropriate. A P value < 0.05 was considered statistically significant.
Data in the Local Health Authority registries are recorded with the patient's consent and can be used as aggregate data for scientific studies without further authorization.18 This study complies with the Declaration of Helsinki and with Italian privacy law.19
A total of 12,288 forms were returned during the study period, but 1,893 (15.4%) were not included in this analysis because the vaccines were not administered according to our observational study inclusion criteria (1,397 received MMR vaccine, alone or with other vaccines; 25 received MMRV with other vaccines; and 471 received V, alone or with other vaccines). We thus assessed 10,395 subjects, 5,130 (49.4%) in the MMR+V group and 5,265 (50.6%) in the MMRV group (Fig. 1).
Figure 1.
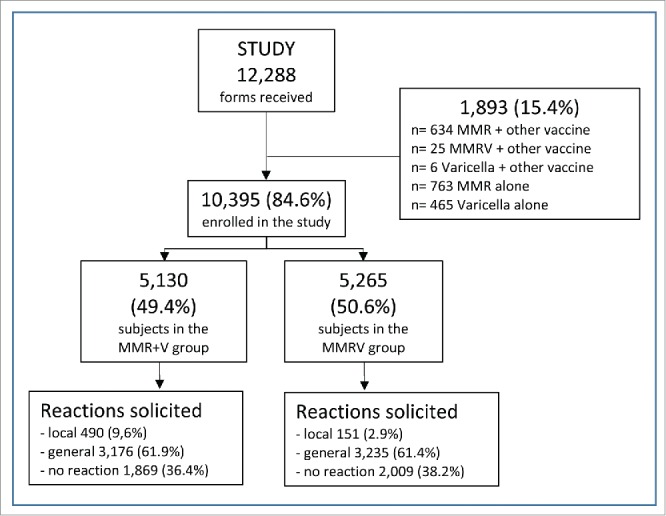
Study flow-chart.
The children's mean age at the time of their vaccination was 14.9±2.3 months (15.0±1.6 months in the MMR+V group, and 14.9±2.7 months in the MMRV group); 51.2% of them were male (50.7% and 51.7% in the MMR+V and MMRV groups, respectively) and the majority (89.7%) were Caucasian. There were no statistically significant differences between the 2 groups' demographic characteristics.
Overall, in 11,197 signs and symptoms solicited by the vaccinations were reported, and their frequency was similar in the 2 groups (53.3% in the MMR+V group versus 46.7% in the MMRV group); 3,222 (28.8%) were considered unrelated to vaccination (32.8% in the MMR+V group and 24.2% in the MMRV group), with a RR of 1.53, and a 95% CI of 1.41–1.66.
The frequency of local reactions to vaccination was significantly lower at the site of injection for the MMRV than for the MMR or varicella vaccines (p<0.01). In general, redness at the injection site was the most frequently reported local reaction in both groups.
Among the general symptoms solicited, fever (of any kind) was the most often reported in both groups. A higher incidence of any fever (defined as an axillary temperature ≥37.5°) was observed in the MMRV group than in the MMR+V group (44.3% vs. 31.6%; RR 1.72; 95% CI: 1.58–1.86). However, the incidence of more severe fever (≥39.5°C) did not differ statistically between the 2 groups, (4.7% after MMRV versus 3.9% after MMR+V) (Table 1).
Table 1.
Numbers and rates of adverse events reported by vaccination group and type of reaction.
| MMR+V (n=5,130) |
MMRV (n=5,265) |
||||
|---|---|---|---|---|---|
| n | (%) | n | (%) | RR (95% CI) | |
| Local reaction | 490 | (9.6) | 151 | (2.9) | 3.33 (2.79–3.98)* |
| pain | 87 | (1.7) | 39 | (0.7) | 2.31 (1.58–3.38)* |
| swelling | 155 | (3.0) | 47 | (0.9) | 3.38 (2.45–4.68)* |
| redness | 296 | (5.8) | 62 | (1.2) | 4.89 (3.73–6.42)* |
| blisters | 64 | (1.2) | 17 | (0.3) | 3.90 (2.28–6.66)* |
| Systemic reaction | 2,567 | (50.0) | 2,736 | (52.0) | 0.96 (0.92–1.00) |
| fever ≤39.4°C | 1,421 | (27.7) | 2,086 | (39.6) | 0.58 (0.54–0.63)* |
| fever ≥39.5°C | 199 | (3.9) | 245 | (4.7) | 0.80 (0.69–1.00) |
| irritability | 655 | (12.8) | 497 | (9.4) | 1.35 (1.21–1.51)* |
| skin blotches and marks | 1,016 | (19.8) | 901 | (17.1) | 1.16 (1.07–1.26)* |
| parotid swelling | 37 | (0.7) | 22 | (0.4) | 1.73 (1.02–2.92)* |
| arthralgia | 55 | (1.1) | 31 | (0.6) | 1.82 (1.17–2.82)* |
| febrile seizures | 7 | (0.14) | 9 | (0.17) | 0.80 (0.30–2.15) |
| no febrile seizures | 2 | (0.04) | 1 | (0.02) | 2.05 (0.18–22.6) |
| unrelated reaction | 690 | (13.5) | 520 | (9.9) | 1.36 (1.22–1.52)* |
| no reaction reported | 1,869 | (36.4) | 2,009 | (38.2) | 0.95 (0.91–1.00) |
p < 0.05
Some kind of rash was reported in 19.8% of subjects in the MMR+V group and in 17.1% in the MMRV group (RR 1.19; 95% CI: 1.08–1.32). All adverse events regressed with no sequelae, without any major medical interventions.
There were a total of 16 cases of febrile seizures (0.15%), with no difference between the 2 groups (0.14% in the MMR+V and 0.17% in the MMRV group). All subjects recovered without sequelae.
At least one drug was administered to 18.6% of the children (and the differences between the 2 groups were not statistically significant). In 87.5% of cases this involved paracetamol (55.1% in the MMRV group vs 44.9% in the MMR+V group; RR 1.24; 95% CI: 1.11–1.38). The family pediatrician was consulted for 2.8% of the children, 0.4% of them underwent laboratory tests, 0.9% were addressed to emergency services, and 0.2% were hospitalized (again with differences between the 2 groups that were not statistically significant).
The Veneto Region is situated in the north-east of Italy where the climate is generally temperate. The population of 4.8 million (9.2% children aged 0–9 years) accounts for 7.7% of the Italian population.
In 2008 the Veneto Regional Authority abolished all mandatory vaccination and, in accordance with legislation suspending mandatory vaccination, it has since undertaken specific projects, including ad hoc software for managing vaccinations, pre-vaccination counseling, adverse event surveillance, training for healthcare workers, the surveillance of vaccine-related disorders, the promotion of healthcare in the first years of life, and the surveillance and prevention of diseases related to travel and immigration. The Veneto's vaccine coverage data reveal a decline in immunization coverage rates for all vaccinations,20 though levels remain above the goal of 95% set by the Italian National Immunization Plan21 (a general decrease has also recently been observed in Italy as a whole).22
This observational cohort study compared the safety of the first dose of MMRV vaccine with that of the MMR+V co-administration in children from the Veneto region. Overall, our study showed that both vaccination strategies are safe and well-tolerated. The method used to actively collect the children's reactions to vaccination demanded considerable resources, but generated useful information on how parents perceive any vaccination-related adverse events. After applying a causal relationship, the number of related AEs dropped significantly in the MMR+V group, confirming that performing more injections increases parents' anxiety because of the perceived additional pain for the infant.23 A higher incidence of fever was observed in the MMRV group than after MMR+V, but this only applied to mild fever, while there was no difference between the 2 groups for severe fever (≥39.5°C axillary temperature). These results are comparable with a recent meta-analysis reporting higher frequencies of fever associated with MMRV.24 This observation is also compatible with the fever peak described after the administration of other vaccines containing measles strains.13
Due to the few episodes of febrile seizures in our sample, our analyses were of limited power for the purpose of assessing the RR of seizures after MMRV vis-à-vis MMR+V. The overall incidence recorded in our analysis was similar to that of another observational study,13 but our data derive from an active surveillance that might increase the frequency of AEs reporting. It is also important to correct the denominator (total number of subjects vaccinated in each of the 2 groups), which is found to be underestimated because parents whose children experience no AEs are less likely to complete and return the form.
This analysis reveals similar safety profiles for the 2 vaccination strategies; an in-depth analysis is needed to evaluate the possible causal relationship between some adverse events reported with incomplete information and vaccination.
The greater risk of febrile seizures with combined quadrivalent vaccines has to be balanced against the potential advantage of a single injection in terms of improving varicella immunization coverage rates.25 This benefit might be important in our present setting, which shows a general decline in vaccination coverage.
In conclusion, our data suggest good safety profiles for both vaccination strategies after the first dose. Healthcare providers should analyze the risks and benefits after providing adequate counseling for guardians before they choose a vaccination strategy. Although the method used to actively record any reactions to vaccination demanded considerable resources, it enabled us to collect important information on the parents' perception of the AEs occurring in response to their child's vaccination.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Klein NP, Lewis E, Fireman B, Hambidge SJ, Naleway A, Nelson JC, Belongia EA, Yih WK, Nordin JD, Hechter RC, et al; Safety of measles-containing vaccines in 1-year-old children. Pediatrics 2015; 135(2):321-9; http://dx.doi.org/ 10.1542/peds.2014-1822 [DOI] [PubMed] [Google Scholar]
- [2].Barlow WE, Davis RL, Glasser JW, Rhodes PH, Thompson RS, Mullooly JP, Black SB, Shinefield HR, Ward JI, Marcy SM, et al; Centers for Disease Control and Prevention Vaccine Safety Datalink Working Group. The risk of seizures after receipt of whole-cell pertussis or measles, mumps, and rubella vaccine. N Engl J Med 2001; 345(9):656-61; PMID:11547719; http://dx.doi.org/ 10.1056/NEJMoa003077 [DOI] [PubMed] [Google Scholar]
- [3].Griffin MR, Ray WA, Mortimer EA, Fenichel GM, Schaffner W. Risk of seizures after measles-mumps-rubella immunization. Pediatrics 1991; 88(5):881-5; PMID:1945626 [PubMed] [Google Scholar]
- [4].Vestergaard M, Hviid A, Madsen KM, Wohlfahrt J, Thorsen P, Schendel D, Melbye M, Olsen J. MMR vaccination and febrile seizures: evaluation of susceptible subgroups and long-term prognosis. JAMA 2004; 292(3):351-7; PMID:15265850; http://dx.doi.org/ 10.1001/jama.292.3.351 [DOI] [PubMed] [Google Scholar]
- [5].Shinefield H, Black S, Digilio L, Reisinger K, Blatter M, Gress JO, Brown ML, Eves KA, Klopfer SO, Schödel F, et al.. Evaluation of a quadrivalent measles, mumps, rubella and varicella vaccine in healthy children. Pediatr Infect Dis J 2005; 24(8):665-9; PMID:16094217; http://dx.doi.org/ 10.1097/01.inf.0000172902.25009.a1 [DOI] [PubMed] [Google Scholar]
- [6].Marin M1, Broder KR, Temte JL, Snider DE, Seward JF; Centers for Disease Control and Prevention (CDC). Use of combination measles, mumps, rubella, and varicella vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2010; 59(RR-3):1-12; PMID:20448530 [PubMed] [Google Scholar]
- [7].Centers for Disease Control and Prevention Prevention of varicella: recommendations of the advisory committee on immunization practices (ACIP). Centers for Disease Control and Prevention. MMWR Recomm Rep 1996; 45:1-36 [PubMed] [Google Scholar]
- [8].Baldo V, Baldovin T, Russo F, Busana MC, Piovesan C, Bordignon G, Giliberti A, Trivello R. Varicella: epidemiological aspects and vaccination coverage in the Veneto Region. BMC Infect Dis 2009; 150:2334-9; PMID:19116339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Baldo V, Ferro A, Napoletano G, Milani S, Bertoncello L, Baldovin T, Trivello R. Universal varicella vaccination in the Veneto Region, Italy: launch of a programme targeting all children aged 14 months and susceptible adolescents. Euro Surveill 2007; 12:E071101.3; PMID:17997908 [DOI] [PubMed] [Google Scholar]
- [10].Bechini A, Boccalini S, Baldo V, Cocchio S, Castiglia P, Gallo T, Giuffrida S, Locuratolo F, Tafuri S, Martinelli D, et al.. Impact of universal vaccination against varicella in Italy. Hum Vaccin Immunother 2015; 11:63-71; PMID:25483517; http://dx.doi.org/ 10.4161/hv.34311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Schink T, Holstiege J, Kowalzik F, Zepp F, Garbe E. Risk of febrile convulsions after MMRV vaccination in comparison to MMR or MMR+V vaccination. Vaccine 2014; 32:645-50; PMID:24374498; http://dx.doi.org/ 10.1016/j.vaccine.2013.12.011 [DOI] [PubMed] [Google Scholar]
- [12].Jacobsen SJ, Ackerson BK, Sy LS, Tran TN, Jones TL, Yao JF, Xie F, Cheetham TC, Saddier P. Observational safety study of febrile convulsion following first dose MMRV vaccination in a managed care setting. Vaccine 2009; 27:4656-61; PMID:19520201; http://dx.doi.org/ 10.1016/j.vaccine.2009.05.056 [DOI] [PubMed] [Google Scholar]
- [13].Ma SJ, Xiong YQ, Jiang LN, Chen Q. Risk of febrile seizure after measles-mumps-rubella-varicella vaccine: A systematic review and meta-analysis. Vaccine 2015; 33(31):3636-49; http://dx.doi.org/ 10.1016/j.vaccine.2015.06.009. [DOI] [PubMed] [Google Scholar]
- [14].Approvazione Nuovo “Calendario Vaccinale della Regione del Veneto”. Delibera della giunta regionale n. 1564 del 26/08/2014. Bollettino ufficiale della Regione Veneto 2014; n.89 del 12/09/2014. [Google Scholar]
- [15].Ministero della Salute Nuovo modello di segnalazione di reazione avversa a farmaci e vaccini. Decreto ministeriale del 2.12. 2003. Gazzetta Ufficiale della Repubblica Italiana 2004; 36:8-15 [Google Scholar]
- [16].Zanoni G, Nguyen TM, Valsecchi M, Gallo G, Tridente G. Prevention and monitoring of adverse events following immunization: the “Green Channel” of the Veneto region in Italy. Vaccine 2003. 22:194-201; PMID:14615146; http://dx.doi.org/ 10.1016/S0264-410X(03)00566-8 [DOI] [PubMed] [Google Scholar]
- [17].Micheletti F, Moretti U, Tridente G, Zanoni G. Consultancy and surveillance of post-immunisation adverse events in the Veneto region of Italy for 1992-2008. Human Vaccines 2011; 7:234-239; PMID:21301223; http://dx.doi.org/ 10.4161/hv.7.0.14613 [DOI] [PubMed] [Google Scholar]
- [18].Garante per la protezione dei dati personali. Autorizzazione generale al trattamento dei dati personali effettuato per scopi di ricerca scientifica - 1° marzo 2012. Gazzetta Ufficiale della Repubblica Italiana 2012; 72:47-52 [Google Scholar]
- [19].Codice in materia di protezione dei dati personali, Decreto Legislativo 30 giugno 2003, n. 196. Gazzetta Ufficiale della Repubblica Italiana 2003; 174 (S.O. 123):1-208 [Google Scholar]
- [20].Da Re F, Russo F, Verizzi E. Report sull'attività vaccinale dell'anno 2013, copertura vaccinale a 24 mesi (coorte 2011) ; internet. Regione Veneto, Settore Promozione e sviluppo Igiene e sanità pubblica. 2014 July 30; update 2015, Feb 12; cited 2015 Sep 04 Available at http://www.regione.veneto.it/web/sanita/monitoraggio-vaccinazioni. [Google Scholar]
- [21].Ministero della Salute Piano nazionale prevenzione vaccinale 2012-2014; internet. Roma, 2014 November 13; update 2015, February 5; cited 2015 September 04 Availableat http://www.salute.gov.it/imgs/C_17_pubblicazioni_1721_allegato.pdf. [Google Scholar]
- [22].Ministero della Salute Sintesi delle coperture vaccinali in età pediatrica 2000-2013 ; internet. Roma, 2014 August 28; update 2015, August 28; cited 2015 September 04 Availableat http://www.salute.gov.it/portale/documentazione/p6_2_8_3_1.jsp?lingua=italiano&id=20 [Google Scholar]
- [23].Dodd D. Benefits of combination vaccines: effective vaccination on a simplified schedule. Am J Manag Care 2003; 9:S6-12; PMID:12564784 [PubMed] [Google Scholar]
- [24].Leung JH, Hirai HW, Tsoi KK. Immunogenicity and reactogenicity of tetravalent vaccine for measles, mumps, rubella and varicella (MMRV) in healthy children: a meta-analysis of randomized controlled trials. Expert Review of Vaccines 2015; 14:1149-57); PMID:26081133; http://dx.doi.org/ 10.1586/14760584.2015.1057572 [DOI] [PubMed] [Google Scholar]
- [25].Streng A, Liese JG. Decline of varicella vaccination in German surveillance regions after recommendation of separate first-dose vaccination for varicella and measles-mumps-rubella. Vaccine 2014; 32:897-900; PMID:24412300; http://dx.doi.org/ 10.1016/j.vaccine.2013.12.065 [DOI] [PubMed] [Google Scholar]


