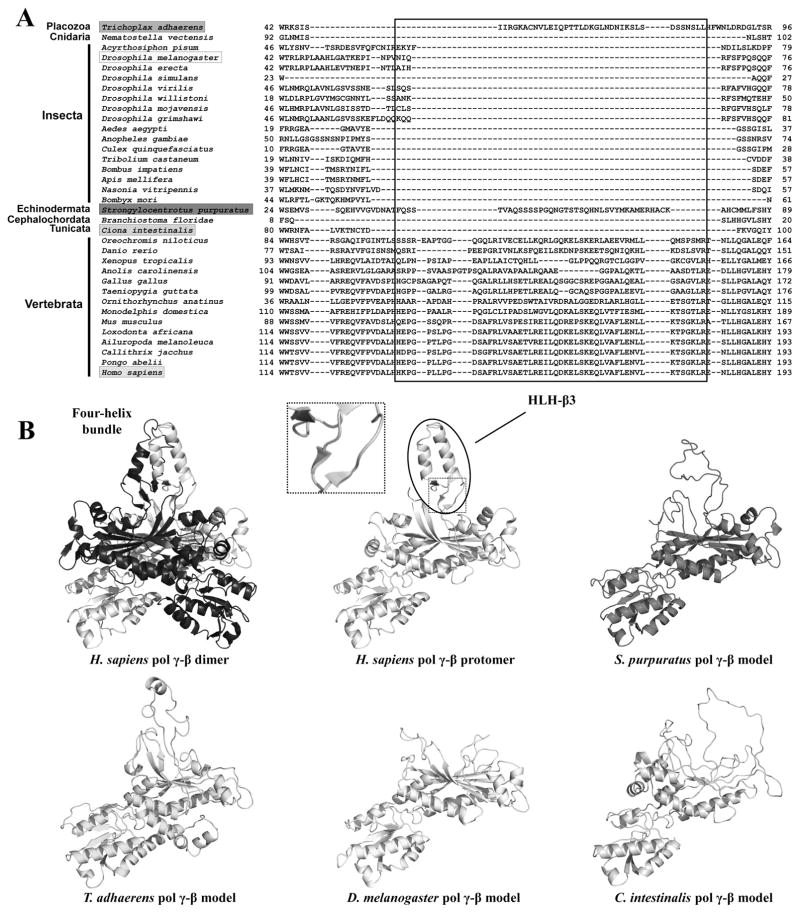
Figure 5.
Dimerization of vertebrate Pol γ-β via the formation of the four-helix bundle structure. A, Amino acid sequence alignment indicates the presence of the HLH-3β domain (boxed) in all species of Vertebrata and possibly in a few other animal groups. B, Comparison of the crystal structure of the human Pol γ-β dimer and structural models for Pol γ-β of Trichoplax adhaerens, Strongylocentrotus purpuratus, Drosophila melanogaster and Ciona intestinalis, showing that only vertebrate Pol γ-β can fold into a HLH-3β structure and therefore, form the four-helix bundle dimerization interface. The inset shows the three short β-sheets at the base of the HLH-3β structure. Reproduced with permission from “M.T. Oliveira, J. Haukka, L.S. Kaguni: Evolution of the metazoan mitochondrial replicase. Genome Biology and Evolution (2015) 7, 943–959”.