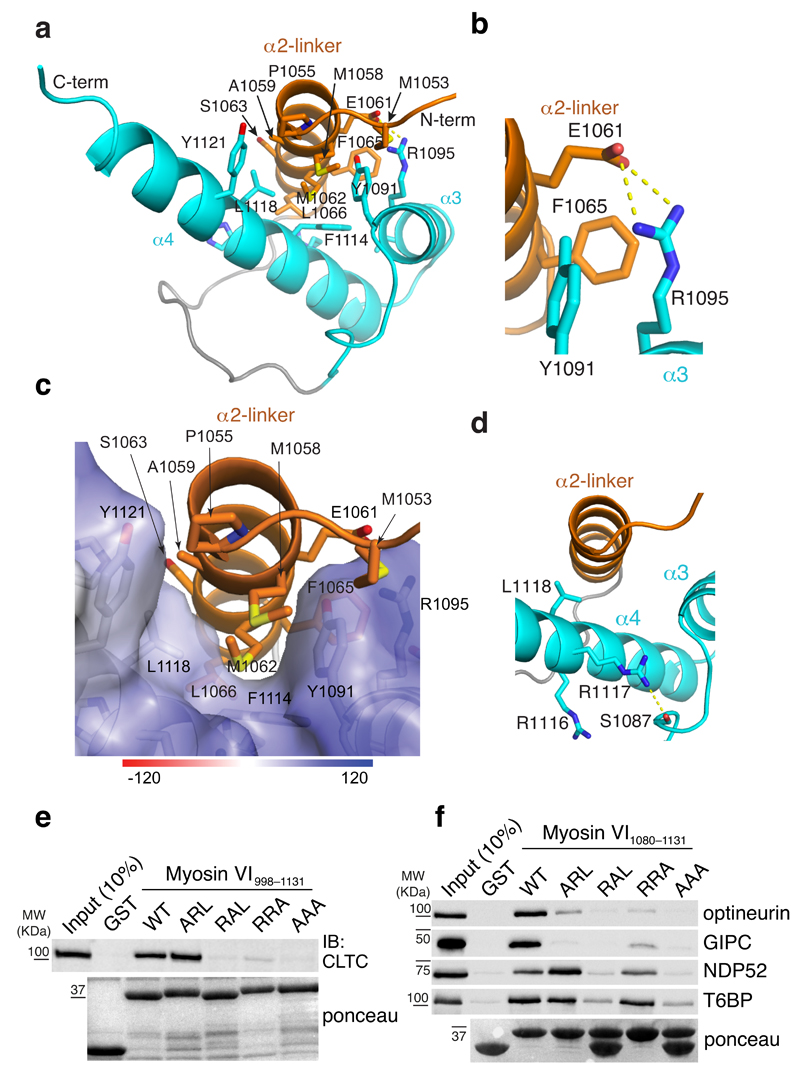
Figure 3. Clathrin interacts specifically with myosin VIlong.
(a) Ribbon representation of the myosin VIlong (R1050–R1131) structure. The isoform-specific α2-linker is in orange while the α3 and α4 helices are displayed in blue. Hydrophobic interactions between the three helices are highlighted. (b) Expanded view of a that illustrates the salt bridge formed between α2-linker E1061 and α4 R1095. (c) Expanded view of a with α2-linker displayed on an electrostatic surface representation of the α3 and α4 helices. (d) Expanded view of a that illustrates the position of the RRL motif residues. L1118 is directed towards α2-linker while R1117 forms a hydrogen bond with S1087. (e,f) GST pull-down assay with myosin VI998–1131 (e) and myosin VI1080–1131 (f) iso3 constructs carrying the indicated mutations in the RRL motif. IB and ponceau as indicated. Except for clathrin (e), tagged constructs were transiently transfected in HEK293T cells and IB were performed with anti-tag antibody.