Figure 2.
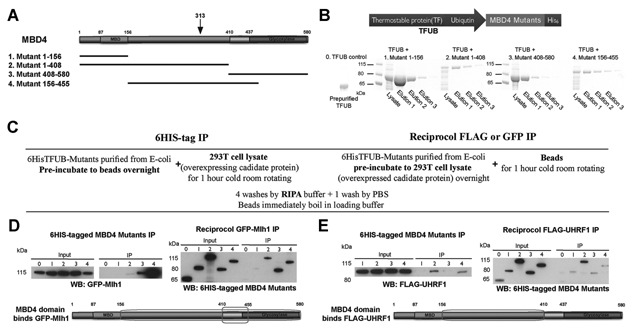
MBD4 interacts with UHRF1 via its intervening region. (A) Schematic representation of the domains of human MBD4 (MBD4 domains are to scale) and the representative scheme of generating the four MBD4 recombinant proteins. A black arrow indicates the natural occurring MBD4 protein truncation site 313 in human MMR‐deficiency carcinomas. (B) The schematic representation of the fusion proteins of MBD4 mutants, and coomaissie staining of the purified MBD4 proteins fused with trigger factor and ubiquitin. Respective total lysates of transformed E.coli cells of MBD4 recombinants 1–4 and their protein elutions are shown as indicated. The dye marker lanes are indicated on the left of lysate lanes with their molecular weight. The empty vector control (labeled as 0. TFUB control) containing TFUB only was from pre‐purified storage, and loaded on the left. The three elutions of individual MBD4 mutants were pooled together and 500 µg of each MBD4 protein mutant was measured for the subsequent reciprocal co‐immunoprecipitation (co‐IP) assays. (C) Flow chart of the reciprocal co‐IP procedure. (D & E) Co‐IP assays to determine the interaction of recombinant MBD4 mutants with Mlh1 (D. control) and UHRF1 (E). Co‐precipitated proteins were detected via western blot. Input (3% of 500 µg GFP‐Mlh1 (D. 6HIS IP) or FLAG‐UHRF1 (E. 6HIS IP), and 3% of 500 µg MBD4 protein mutants in D and E) and the antibody coupled IP are indicated with antibodies used for immunodetection. The migration of the molecular weight is indicated on the left of each blot. The regions labelled by a square on the schematic representation of MBD4 are identified the interacting domains responsible for the associations with Mlh1 (D. control) and UHRF1 (E).
