Figure 4.
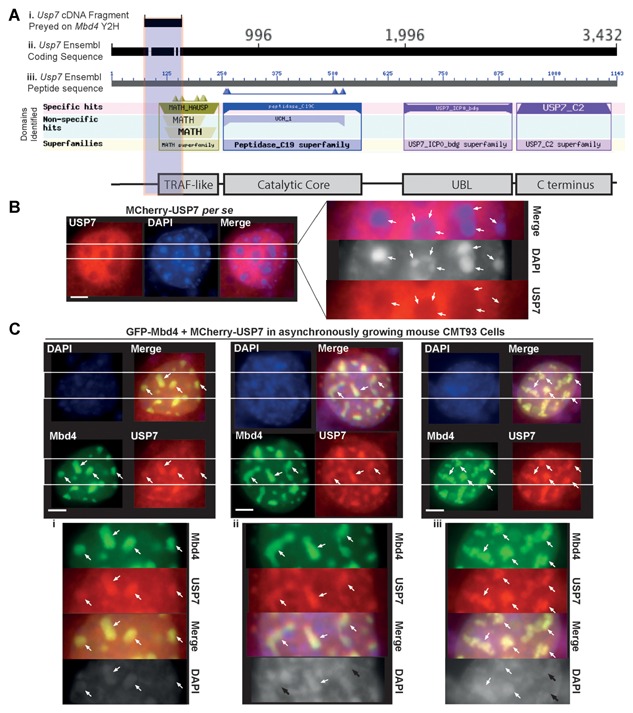
MBD4 directly interacts with and recruits USP7 to chromocenters. (A) The representative scheme for alignment and mapping of a USP7 cDNA fragment that iinteracts with MBD4 in a Y2H screen, (i) USP7 coding sequence, (ii) corresponding peptide sequence, and (iii) schematic representation of domains of USP7. Protein domains are identified by searching Blast integrated SMART domain database (USP7 domains are to scale). The vertical gray shadow indicates the domain region of USP7 overlapping with interacting cDNA fragment. (B & C) Asynchronously growing mouse CMT93 cells were grown on coverslips and transfected by MCherry‐USP7 (B), or co‐transfected by GFP‐MBD4 and MCherry‐USP7 (C). The cells were fixed 48 h later and analyzed directly by IF. Nuclear counterstaining was visualized with DAPI. (B) Distribution of MCherry‐USP7 in CMT93 cells. (C) GFP‐MBD4 recruits MCherry‐USP7 exclusively to chromocenters in all the CMT93 cells co‐transfected. In all the immunostaining images in C, the cells exhibit marked heterochromatin remodeling. The insets on the right (B) or below (C. i, ii, iii) correspond to magnifications of the areas indicated by the two parallel white lines. White arrows indicate triple co‐localization of MBD4, USP7 and DAPI bright spots, while black arrows indicate colocalization of MBD4 and USP7 with diminishing or disappearing DAPI bright spots. Scale bars, 10 µm.
