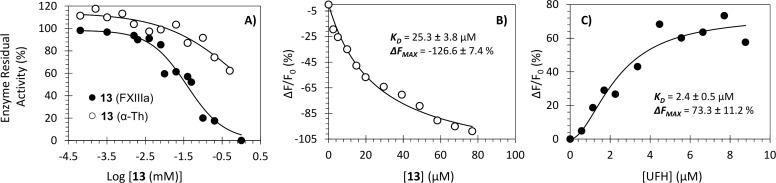
Fig 3. Interaction of human FXIIIa and α-thrombin (α-Th) with NSGM 13 and UFH.
(A) The inhibition of FXIIIa (●) and α-Th (○) by NSGM 13 was measured spectrofluorometrically through a bisubstrate, fluorescence-based transglutamination assay (FXIIIa) or chromogenic substrate assay (α-Th) at pH 7.4/8.0 and 37°C. Solid lines represent sigmoidal fits to the data to obtain IC50, HS, YM, and YO using Eq 1. (B) Spectrofluorometric measurement of the affinity of human FXIIIa for inhibitor 13 at pH 8.0 and 37°C using the intrinsic tryptophan fluorescence (λEM = 348 nm, λEX = 280 nm). Solid lines represent nonlinear regressional fits using quadratic Eq 2. (C) Spectrofluorimetric measurement of the affinity of human FXIIIa for UFH at pH 8.0 and 37°C using the intrinsic tryptophan fluorescence (λEM = 348 nm, λEX = 280 nm). Solid lines represent nonlinear regressional fits using the standard Hill Eq 3. See details in Materials and Methods.