Abstract
Oxidative stress is considered an etiological factor responsible for several symptoms of inflammatory bowel disease (IBD). In vitro anti-inflammatory activities of heat-killed Lactococcus lactis subsp. lactis BF3 have been reported. In this study, the anti-inflammatory effect of these cells was examined using a dextran sodium sulphate (DSS)-induced murine IBD model. Administration of heat-killed L. lactis BF3 via drinking water suppressed the IBD symptoms, such as shortening of colon length, damage to the colon mucosa as observed under the microscope, and spleen enlargement. This result suggests that heat-killed L. lactis BF3 has the potential to treat IBD.
Keywords: Lactococcus lactis, inflammatory bowel disease, mice
Inflammatory bowel disease (IBD) includes ulcerative colitis (UC) and Crohn’s disease (CD). Although IBD is thought to be primarily a condition of young individuals, a significant proportion of new cases of IBD are diagnosed in elderly persons [1]. Recently, it was estimated that over 1 million residents in the USA and 2.5 million residents in Europe have IBD [2]. Moreover, IBD has increased in Asia, South America, and the Middle East including newly industrialized countries [2, 3].
The pathogenesis of IBD is not completely understood, but it is considered that oxidative stress is an etiological factor involved in several signs and symptoms of IBD [4]. Therefore, many studies have focused on describing food materials and extracted compounds with antioxidant activities to be used for IBD treatment. Recently, the amelioration of IBD by some lactic acid bacteria (LAB), owing to their antioxidant and anti-inflammatory effects has also been studied [5, 6]. Previously, we isolated LABs from the intestine of chum salmon (Oncorhynchus keta) that were caught in the sea near Rausu, in Japan. Among the LAB isolates, Lactococcus lactis subsp. lactis BF3 showed higher acid, bile, and salt resistances compared with those of the type strain [7]. Furthermore, heat-killed cells of L. lactis BF3 showed a protective effect in eukaryotic cells, protecting them from hydrogen peroxide toxicity and the inhibitory effect of E. coli lipopolysaccharide (LPS)-induced nitric oxide (NO) secretion in murine macrophage RAW264.7 cells. These results suggest that not only live cells but also heat-killed cells of L. lactis BF3 have potential to be used for IBD treatment through stress resistance and antioxidant and anti-inflammatory activities. However, these effects of L. lactis BF3 have not been confirmed in vivo. In this study, to verify the effectiveness of heat-killed L. lactis BF3 against IBD, the anti-inflammatory effect of these cells in a dextran sodium sulphate (DSS)-induced murine model of IBD was examined.
L. lactis BF3 (accession No. AB973593) isolated from Oncorhynchus keta was stored in a Microbank (Iwaki Co., Tokyo, Japan) at −80°C [7]. Before examination, the frozen strains were thawed and pre-cultured in de Man, Rogosa and Sharpe (MRS) broth (Oxoid, Basingstoke, UK) at 37°C for 24 hr. The bacterial cells were washed with PBS three times, suspended in distilled water, adjusted to an OD660 of 10 (about 109 colony forming units (CFU)/ml), and subjected to heat treatment in boiling water for 20 min. The prepared cell suspension was stored at 4°C and used within 3 days.
This animal experiment was performed in compliance with the fundamental guidelines for proper conduct of animal experiments and related activities in academic research institutions under the jurisdiction of the Ministry of Education, Culture, Sports, Science and Technology of Japan. It was approved by the animal experiment committee of the Tokyo University of Marine Science and Technology (Approval No. H27-4).
Eighteen 5-week-old male ddY mice were obtained from Tokyo Laboratory Animals Science (Tokyo, Japan). The mice were acclimatized in a negative pressure rack maintained at 20–24°C, with a relative humidity of 50–60%, and they were fed a CE2 diet (CLEA Japan, Tokyo, Japan) and distilled water. After 7 days, the mice were divided into 3 groups (n=6). Among them, the untreated control (control) and DSS control groups were fed the same diet and distilled water. The BF3 treated group was fed the same diet but was fed the prepared L. lactis BF3 cell suspension instead of drinking water. After 3 days, 5% (w/v) DSS (MW = 5000; Wako Pure Chemical Industries, Osaka, Japan) was added to the drinking water of the DSS control and BF3-treated groups. The mice received diet and water ad libitum.
After 7 days of DSS administration, the mice were anesthetized with diethyl ether and exsanguinated from the abdominal aorta. The large intestine (colon) was excised and washed with PBS, and then its length was measured. The severity of IBD was also evaluated on the basis of histological observations of hematoxylin and eosin (HE)-stained tissues of the colon [6]. Approximately 5-mm-long sections of the middle section of the colon were soaked in 10% formalin to prepare samples for microscopic analysis and HE staining (MedRidge, Tokyo, Japan). The liver, spleen, and kidneys were also excised and weighed as indices of DSS toxicity [8, 9].
Data are presented as the mean ± SEM. Data from experiments were analyzed by ANOVA and Tukey’s test using a statistical software (Excel Statistic Ver. 6, Esumi, Tokyo, Japan).
To determine the anti-inflammatory effect of heat-killed L. lactis BF3 on IBD, 5% (w/v) DSS in drinking water was administered to mice with or without treatment of L. lactis BF3. As shown in Table 1, after 7 days of DSS treatment, the body weights in the DSS control group tended to be lower than those in the control group. This effect was tended to be suppressed by the LAB cells. At that time, diarrhea and bloody bowel discharge were observed only in mice of the DSS control group.
Table 1. Body and organ weights of the experimental mice.
| Control | DSS control | BF3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Body weight (g) | |||||||||
| Initial | 29.7 | ± | 0.6 | 30.1 | ± | 0.5 | 30.3 | ± | 0.5 |
| 3 days after BF3 administration | 31.2 | ± | 0.4 | 31.8 | ± | 0.7 | 31.6 | ± | 0.6 |
| 7 days after DSS administration | 33.7 | ± | 0.5 | 30.8 | ± | 1.2 | 31.7 | ± | 1.0 |
| Organ weight (g) | |||||||||
| Liver | 1.916 | ± | 0.041a | 1.414 | ± | 0.082b | 1.580 | ± | 0.082b |
| Kidneys | 0.515 | ± | 0.016 | 0.510 | ± | 0.018 | 0.513 | ± | 0.026 |
| Spleen | 0.117 | ± | 0.098b | 0.244 | ± | 0.046a | 0.146 | ± | 0.015ab |
Values are shown as the mean and SEM (n=6).
Means within each SEM having different letters are significantly different (p<0.05).
DSS treatment decreased the liver weight from about 1.916 g to 1.414 g. This effect was also suppressed by L. lactis BF3. There was no significant effect on kidney weight. The weight of the spleen of mice in the DSS control group was about two times higher than that of the control group mice. The spleen enlargement also tended to be supressed by L. lactis BF3. Enlargement of the spleen, an organ of the immune system, caused by the administration of DSS has been previously reported [6, 9].
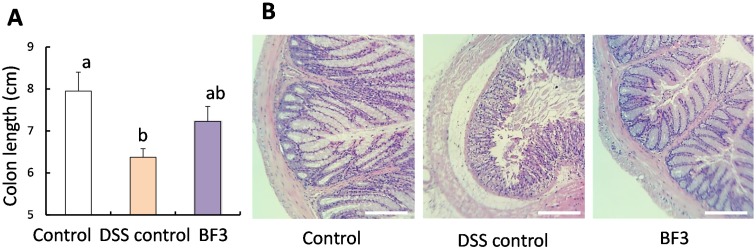
As shown in Fig. 1A, the colon length was shorter in mice in the DSS control group compared with that observed in the control group mice. This represents the index of inflammation caused by IBD [10]. However, treatment with L. lactis BF3 resulted in a recovery of colon length by approximately 50% compared with the DSS control group. This result indicates that L. lactis BF3 prevented IBD induced by DSS. Fig. 1B shows typical images of HE-stained colon tissue. In the control group, the sections of the crypt structure in the mucosal layer, the submucosa, and muscular layer were normal. In the DSS control group, the crypt structure and submucosa were irregular. These irregularities caused by DSS were suppressed by treatment with L. lactis BF3.
Fig. 1.
Colon length (A) and images of hematoxylin and eosin (HE)-stained colons (B) of mice that drank distilled water (control), distilled water and 5% (w/v) DSS (DSS control), or DSS with distilled water containing heat-killed cells of L. lactis BF3 (BF3).
Values in (B) are expressed as the mean ± SE (n=6). Means within each error bar having different letters are significantly different (p<0.05). Scale bars=0.25 mm.
In many studies of anti-inflammatory effects on macrophage cells and enterocytes, the heat-killed LAB cells have been used [6, 7, 11, 12]. On the other hand, the anti-inflammatory effects of live cells on a DSS-induced murine model of IBD have also been reported by many researchers [13,14,15]. It is thought that it may be better to use heat-killed LAB cells, as they may be more stable and safer than live cells [16]. It was previously reported that heat treatment denatured the cell membrane and outer cell compounds [17]. In some cases, the denatured cells do perform some functions as well as or better than live cells. For examples, Li et al. [16] reported that both live and heat-killed Lactobacillus rhamnosus GG provided suppressed LPS-induced proinflammatory mediators and increased anti-inflammatory mediators. Furthermore the in vitro bile acid binding capacities of cells of Lactobacillus plantarum, L. lactis, and Leuconostoc mesenteroides strains isolated from fish intestines were increased by heating [18]. The findings of this study suggest that the heat-killed cells of L. lactis BF3 also have the potential for anti-IBD therapy. Studies on functional compounds such as exopolysaccharides, and the mechanisms of the reported anti-IBD effect of live and heat-killed L. lactis BF3 are currently in progress.
REFERENCES
- 1.Gisbert JP, Chaparro M. 2014. Systematic review with meta-analysis: inflammatory bowel disease in the elderly. Aliment Pharmacol Ther 39: 459–477. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan GG. 2015. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol 12: 720–727. [DOI] [PubMed] [Google Scholar]
- 3.Hu PJ. 2015. Inflammatory bowel disease in Asia: the challenges and opportunities. Intest Res 13: 188–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moura FA, de Andrade KQ, dos Santos JCF, Araújo ORP, Goulart MOF. 2015. Antioxidant therapy for treatment of inflammatory bowel disease: does it work? Redox Biol 6: 617–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Satish Kumar CS, Kondal Reddy K, Reddy AG, Vinoth A, Ch SR, Boobalan G, Rao GS. 2015. Protective effect of Lactobacillus plantarum 21, a probiotic on trinitrobenzenesulfonic acid-induced ulcerative colitis in rats. Int Immunopharmacol 25: 504–510. [DOI] [PubMed] [Google Scholar]
- 6.Kawahara M, Nemoto M, Nakata T, Kondo S, Takahashi H, Kimura B, Kuda T. 2015. Anti-inflammatory properties of fermented soy milk with Lactococcus lactis subsp. lactis S-SU2 in murine macrophage RAW264.7 cells and DSS-induced IBD model mice. Int Immunopharmacol 26: 295–303. [DOI] [PubMed] [Google Scholar]
- 7.Kuda T, Noguchi Y, Ono M, Takahashi H, Kimura B, Kamita R, Eto T, Kato M, Kawahara M. 2014. In vitro evaluation of the fermentative, antioxidant, and anti-inflammation properties of Lactococcus lactis subsp. lactis BF3 and Leuconostoc mesenteroides subsp. mesenteroides BF7 isolated from Oncorhynchus keta intestines in Rausu, Japan. J Funct Foods 11: 269–277. [Google Scholar]
- 8.Karlsson A, Jägervall A, Pettersson M, Andersson AK, Gillberg PG, Melgar S. 2008. Dextran sulphate sodium induces acute colitis and alters hepatic function in hamsters. Int Immunopharmacol 8: 20–27. [DOI] [PubMed] [Google Scholar]
- 9.Dranse HJ, Rourke JL, Stadnyk AW, Sinal CJ. 2015. Local chemerin levels are positively associated with DSS-induced colitis but constitutive loss of CMKLR1 does not protect against development of colitis. Physiol Rep 3: e12497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ito R, Shin-Ya M, Kishida T, Urano A, Takada R, Sakagami J, Imanishi J, Kita M, Ueda Y, Iwakura Y, Kataoka K, Okanoue T, Mazda O. 2006. Interferon-gamma is causatively involved in experimental inflammatory bowel disease in mice. Clin Exp Immunol 146: 330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Xie J, Wang N, Li Y, Sun X, Zhang Y, Zhang H. 2013. Lactobacillus casei Zhang modulate cytokine and toll-like receptor expression and beneficially regulate poly I:C-induced immune responses in RAW264.7 macrophages. Microbiol Immunol 57: 54–62. [DOI] [PubMed] [Google Scholar]
- 12.Fang SB, Shih HY, Huang CH, Li LT, Chen CC, Fang HW. 2014. Live and heat-killed Lactobacillus rhamnosus GG upregulate gene expression of pro-inflammatory cytokines in 5-fluorouracil-pretreated Caco-2 cells. Support Care Cancer 22: 1647–1654. [DOI] [PubMed] [Google Scholar]
- 13.Watterlot L, Rochat T, Sokol H, Cherbuy C, Bouloufa I, Lefèvre F, Gratadoux JJ, Honvo-Hueto E, Chilmonczyk S, Blugeon S, Corthier G, Langella P, Bermúdez-Humarán LG. 2010. Intragastric administration of a superoxide dismutase-producing recombinant Lactobacillus casei BL23 strain attenuates DSS colitis in mice. Int J Food Microbiol 144: 35–41. [DOI] [PubMed] [Google Scholar]
- 14.Toumi R, Soufli I, Rafa H, Belkhelfa M, Biad A, Touil-Boukoffa C. 2014. Probiotic bacteria lactobacillus and bifidobacterium attenuate inflammation in dextran sulfate sodium-induced experimental colitis in mice. Int J Immunopathol Pharmacol 27: 615–627. [DOI] [PubMed] [Google Scholar]
- 15.Chauhan R, Vasanthakumari AS, Panwar H, Mallapa RH, Duary RK, Batish VK, Grover S. 2014. Amelioration of colitis in mouse model by exploring antioxidative potentials of an indigenous probiotic strain of Lactobacillus fermentum Lf1. Biomed Res Int 2014: 206732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li N, Russell WM, Douglas-escobar M, Hauser N, Lopez M, Neu J. 2009. Live and heat-killed Lactobacillus rhamnosus GG: effects on proinflammatory and anti-inflammatory cytokines/chemokines in gastrostomy-fed infant rats. Pediatr Res 66: 203–207. [DOI] [PubMed] [Google Scholar]
- 17.Lee J, Kaletunç G. 2002. Evaluation of the heat inactivation of Escherichia coli and Lactobacillus plantarum by differential scanning calorimetry. Appl Environ Microbiol 68: 5379–5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuda T, Masuko Y, Kawahara M, Kondo S, Nemoto M, Nakata T. 2016. Bile acid-lowering properties of Lactobacillus plantarum Sanriku-SU3 isolated from Japanese surfperch fish. Food Biosci 14: 41–46. [Google Scholar]