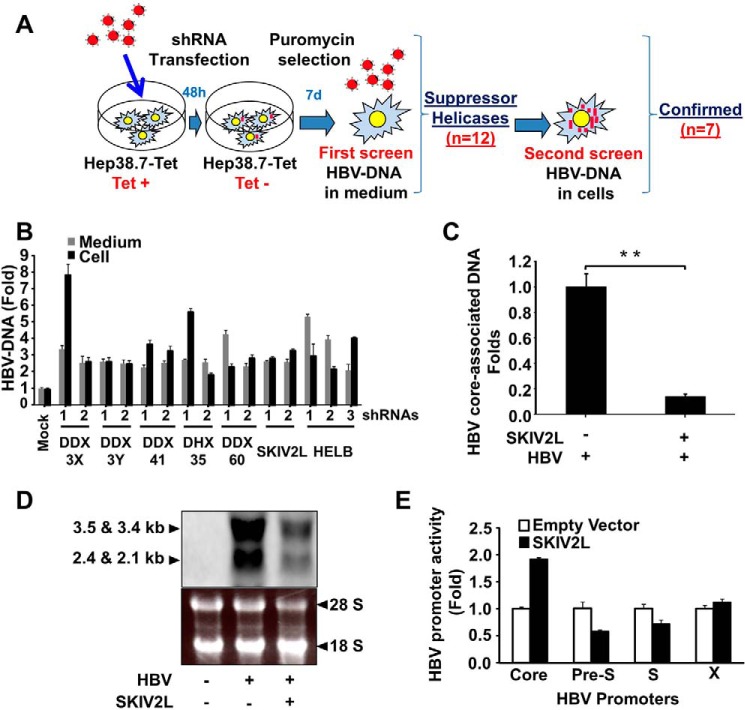
FIGURE 1.
SKIV2L suppresses HBV replication. A, Diagram representing the screening approach. B, helicases that suppress HBV replication. Two or more different shRNAs targeting the genes encoding DDX3X, DDX3Y, DDX41, DHX35, DDX60, SKIV2L, or HelB helicases (or non-targeting control shRNAs) were transfected into Hep38.7-Tet (+) cells 2 days before tetracycline withdrawal, and stable transfectants were selected using puromycin. After 1 week of selection, HBV-DNA titers were quantified (separately) in the cells and medium using real-time PCR. Titers were plotted relative to those obtained in the control cells (transfected with non-targeting shRNAs). Three independent experiments were performed in triplicate, and data are presented as the mean ± S.D. C, HepG2 cells were co-transfected with HBV-D60 together with pEF4-SKIV2L-MycHis or empty vector (pEF4-MycHis). Four days after transfection, HBV core-associated DNA was quantified by real-time PCR. Data are plotted as -fold difference compared with mock-treated cells. Three independent experiments were performed in triplicate, and data are presented as the mean ± S.D. D, HepG2 cells were co-transfected with HBV-D60 plasmid or empty vector (pUC19; mock) together with pEF4-SKIV2L-MycHis or pEF4-MycHis. Three days later intracellular HBV-RNA levels were assessed by Northern blot. Northern blot analysis of HBV-RNA is shown in the upper panel. The lower panel shows the cellular ribosomal RNA stained with ethidium bromide. E, firefly luciferase reporter plasmids harboring HBV promoters (or no insert), Renilla luciferase reporter plasmids, and either SKIV2L or empty vector constructs were co-transfected into HepG2 cells. Dual luciferase assays were performed at 48 h after transfection. Relative luciferase units are plotted as -fold difference relative to mock-treated cells. Three independent experiments were performed in triplicate, and data are presented as the mean ± S.D. Three independent experiments were performed in triplicate, and data are presented as the mean ± S.D. Statistical significance was measured using two-tailed Student's t test. ** = p < 0.01.