FIGURE 9.
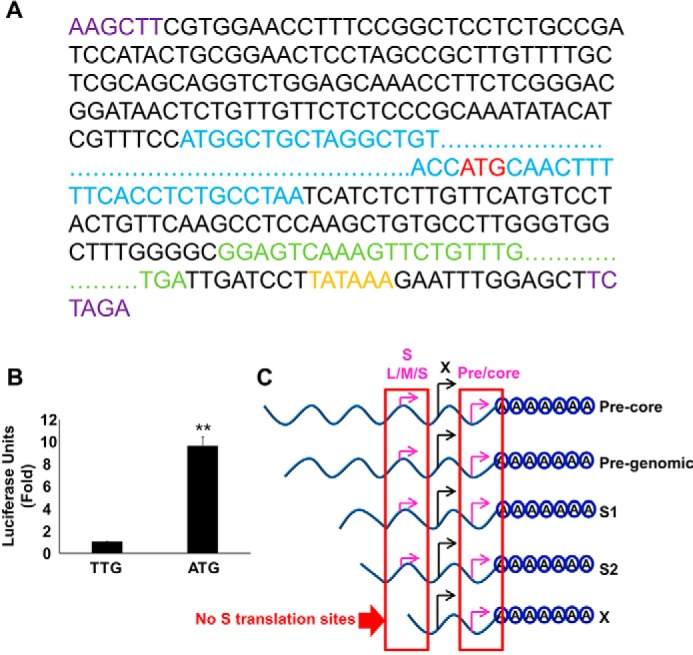
Translation from the 3′ precore regulates the stability of HBV X-mRNA. A, schematic of the HBV X mRNA-Gaussia luciferase (GLuc) coding sequence translation fusion. The GLuc sequence (green) was inserted in place of the redundant core sequence corresponding to the 3′-end of the X mRNA; a HBV-poly(A) signal (orange) also was inserted after the GLuc coding sequence. The ATG start codon (red) of the 3′ precore was left intact or was mutated to TTG (to prevent possible translation from this site). This entire sequence then was subcloned (via flanking HINDIII and XBAI sites (purple)) into the pcDNA3.1 vector, placing the construct under the control of the CMV promoter. Because the GLuc coding sequence is only in-frame with the 3′ precore ATG start codon, GLuc expression should result only if the ribosome skips the first ATG (X ORF) and starts translation at the 3′ precore ATG start codon (red). The X protein ORF is shown in blue. For clarity, extended sequences are not shown and are instead indicated by dots. B, the 3′ precore translation initiation site is active in HBV X-mRNA. HepG2 cells were transfected with either pcDNA3.1-X (ATG-GLuc) or pcDNA3.1-X (TTG-GLuc) plasmids containing the GLuc sequence in frame with an active (ATG) or mutated (TTG) 3′ precore translation start codon, respectively. 48 h later GLuc activity was measured in the medium and plotted as -fold difference relative to the activity from pcDNA3.1-X (TTG-GLuc)-transfected cells. Three independent experiments were performed in triplicate, and the data are was presented as the mean ± S.D. C, a diagram showing the in-frame sequence of HBV-3′ precore and HBV-S (L/M/S) start codons; as a result of this translation is only expected to be initiated from the HBV-3′ precore in the X transcript because it lacks the S (L/M/S) start codons. Statistical significance was measured was measured using two-tailed Student's t test. ** = p < 0.01.
