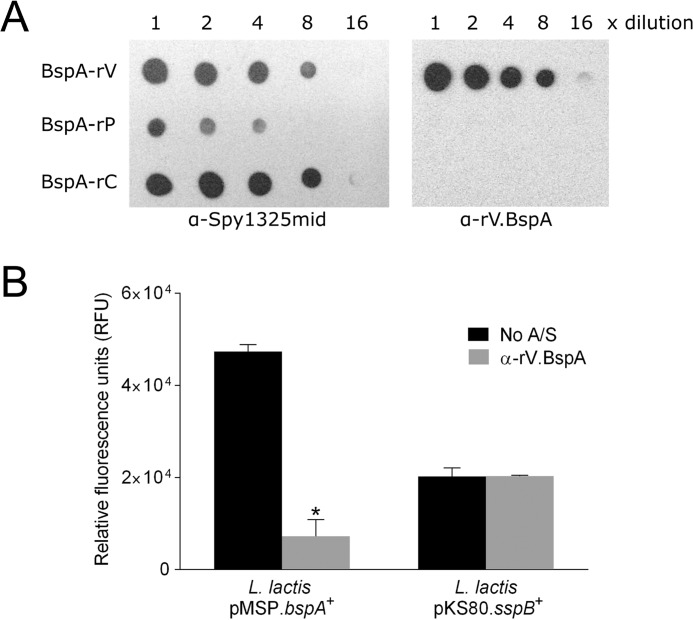
FIGURE 3.
Antibody inhibition binding studies. A, affinity purification of α-rV.BspA antibodies. Antibodies reactive against recombinant BspA-V (α-rV.BspA) were affinity-purified from antibodies raised against AspA (α-Spy1325mid). Purified α-rV.BspA antibodies were used to probe dot immunoblots of serially diluted (1:2) recombinant proteins corresponding to BspA-V, -P, and -C domains. Purified antibodies reacted only with BspA-rV. B, effects of affinity-purified antibody α-rV.BspA on adherence of L. lactis expressing BspA to immobilized gp340. L. lactis pMSP.bspA+ cells were FITC-labeled, preincubated without antibody or with 2.4 μg α-rV.BspA, and then incubated in microwells coated with 50 ng of gp340 for 2 h at 37 °C. Nonadherent cells were removed, and relative numbers of attached cells measured in a fluorescence plate reader. L. lactis pKS80.sspB+ expressing S. gordonii SspB was included as a control. Values given represent the mean ± S.D. of two independent experiments performed in duplicate. *, p < 0.0001 relative to no antiserum (A/S) control, calculated using an unpaired Student's t test.