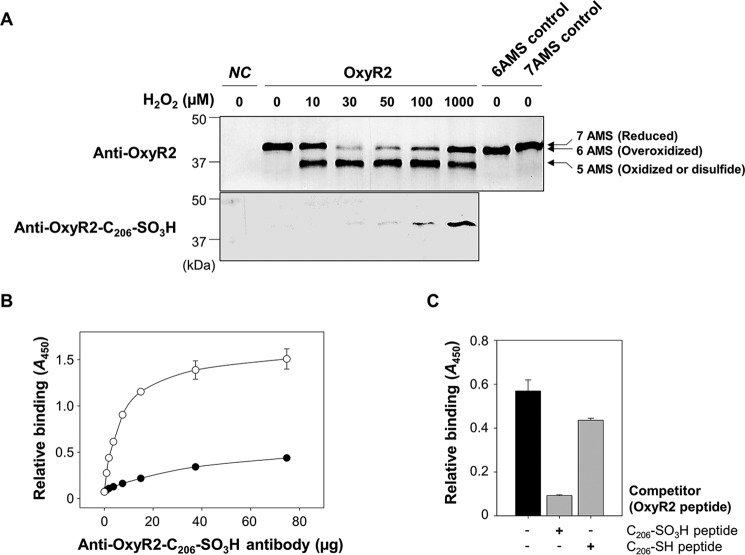
FIGURE 2.
Overoxidation of OxyR2 Cys206 in V. vulnificus cells exposed to various levels of H2O2. A, OH0703 (pDY1025) was grown anaerobically to an A600 of 0.3 and exposed to various H2O2 concentrations for 3 min. Cellular proteins were precipitated with TCA and alkylated with fresh AMS buffer for 1 h at 37 °C. Proteins (3.5 μg for the top panel and 7 μg for the bottom panel) were resolved by non-reducing SDS-PAGE and immunoblotted using anti-OxyR2 antibody (top) or anti-OxyR2-Cys206-SO3H antibody (bottom). The predicted numbers of AMS that alkylated each OxyR2 molecule and their redox states are indicated at the ends of the gel. Negative control (NC) was OH0703 (pJH0311), 6AMS control was OH0703 (pBANG1416), and 7AMS control was OH0703 (pDY1025). B, the specificity of anti-OxyR2-Cys206-SO3H antibody to overoxidized and reduced OxyR2 was determined using ELISA. The microtiter 96-well plates were coated with 0.1 μg of synthetic peptides corresponding to either OxyR2 active site with overoxidized (S-sulfonated) Cys206 (EKEHC206(SO3H)LTEHA) (○) or with reduced Cys206 (EKEHC206(SH)LTEHA) (●), and the peptides were reacted with various concentrations of the antibody as indicated. C, the S-sulfonated OxyR2 peptide (0.1 μg) was attached to the microtiter 96-well plates and then reacted with 4 μg of anti-OxyR2-Cys206-SO3H antibody. As a binding competitor, either S-sulfonated or reduced OxyR2 peptides (12.5 ng) were added to the reaction with the antibody as indicated. Black bar, control where no competitor was added. Relative binding of the antibody to the specific peptides is presented as A450. All data in B and C represent mean ± S.D. (error bars).