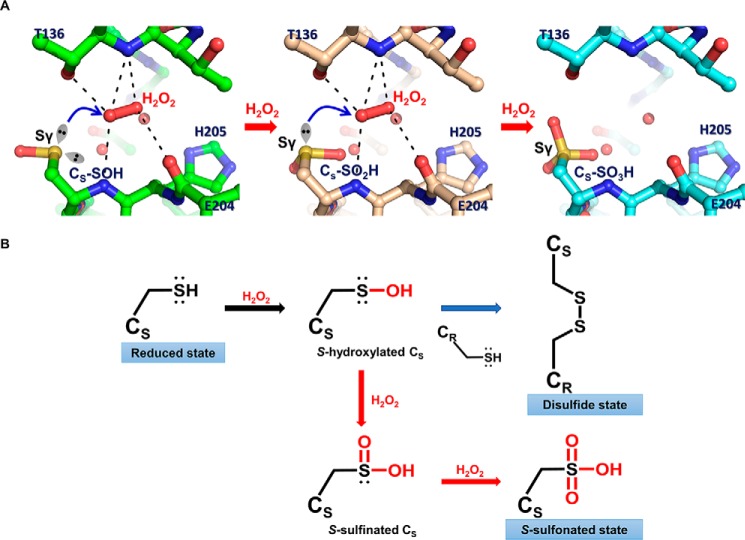
FIGURE 6.
Proposed overoxidation mechanism of OxyR. A, modeled structures of OxyR, focusing on the sensing cysteine (CS). S-Hydroxylated sensing cysteine (CS-SOH) is oxidized by the bound H2O2 (left), resulting in S-sulfinated sensing cysteine (CS-SO2H; middle), which is in turn further oxidized to S-sulfonated sensing cysteine (CS-SO3H; right). The models were constructed based on the crystal structure of the P. aeruginosa OxyR-C199D variant complexed with a H2O2 molecule. The H2O2 molecule was bound by the polar interactions with the side chain and the backbone amide group of Thr136, the backbone carbonyl group of Glu204, and the backbone amide group of Cys206, as indicated by the dotted lines. The lone pair of electrons are displayed as the sp3 orbital shapes, and the bound water molecules are shown as red spheres. The blue arrows indicate the nucleophilic attack by Sγ of sensing cysteine on an oxygen atom of H2O2. B, schematic reaction mechanism of oxidation of sensing cysteine and the concomitant three redox states of OxyR. Sensing cysteine of OxyR in the reduced state is converted to S-hydroxylated sensing cysteine by H2O2, as a reaction intermediate (black arrow). S-Hydroxylated sensing cysteine rapidly makes a disulfide with the other redox-sensitive cysteine (CR), resulting in the disulfide state of OxyR (blue arrow). Alternatively, S-hydroxylated sensing cysteine is further oxidized consecutively by H2O2 to the final S-sulfonated sensing cysteine in the presence of excess H2O2, resulting in the overoxidized state of OxyR (red arrows).