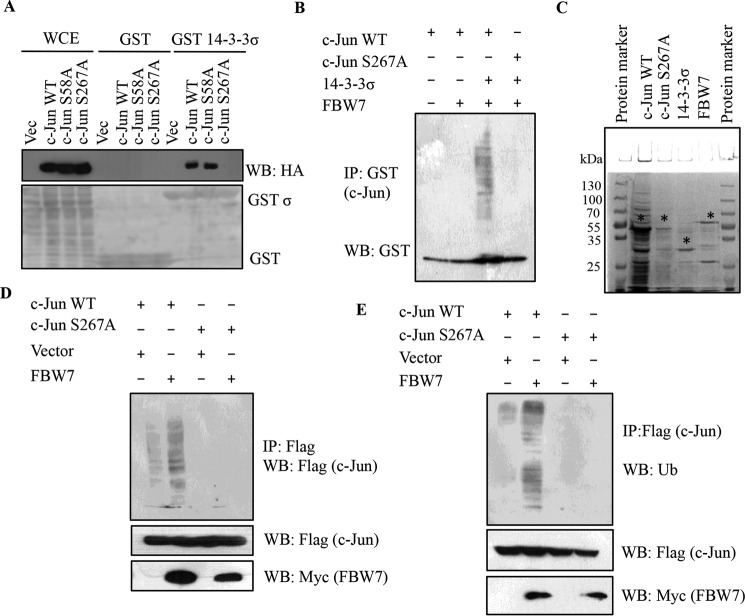
FIGURE 6.
14-3-3σ targets c-Jun to proteasomal degradation in an FBW7-dependent manner. A, HEK293 cells were transfected with HA-c-Jun WT, HA-c-Jun S58A, and HA-c-Jun S267A. 24 h post-transfection, cell lysates were prepared and incubated with bacterially purified GST or GST-14-3-3σ, and the reactions were resolved by SDS-PAGE. 5% input for whole cell extract (WCE) served as the input. Western blotting was performed with the indicated antibodies (upper panel), and the levels of GST and GST-14-3-3σ are shown in the Ponceau-stained membrane (lower panel). B, in vitro ubiquitination experiments were performed using bacterially purified GST-c-Jun WT and GST-c-Jun S267A as substrates in the indicated combinations with MBP-tagged 14-3-3σ and MBP-tagged FBW7α along with E1 (UBE1) and E2 (UbcH5B). Full-length and ubiquitinated species of GST-c-Jun WT and GST-c-Jun S267A were detected by immunoblotting with anti-GST antibody. C, the protein levels of GST-c-Jun WT, GST-c-Jun S267A, MBP-14-3-3σ, and MBP-FBW7α shown by Coomassie staining. The * indicates the full-length protein; the lower molecular bands are probably degradation products. D, 14-3-3σ+/+ cells were transfected with FLAG-tagged c-Jun WT and c-Jun S267A with or without Myc-FBW7. 24 h post-transfection, cells were treated with MG132 for 6 h, and immunoprecipitated with anti-FLAG antibody. c-Jun ubiquitination was detected by immunoblotting with FLAG antibody. E, 14-3-3σ+/+ cells were transfected with FLAG-tagged c-Jun WT and c-Jun S267A with or without Myc-FBW7. At 24 h post-transfection, cells were treated with MG132 for 6 h, and immunoprecipitated with anti-FLAG antibody. c-Jun ubiquitination was detected by immunoblotting with ubiquitin (Ub) antibody. IP, immunoprecipitation; WB, Western blotting.