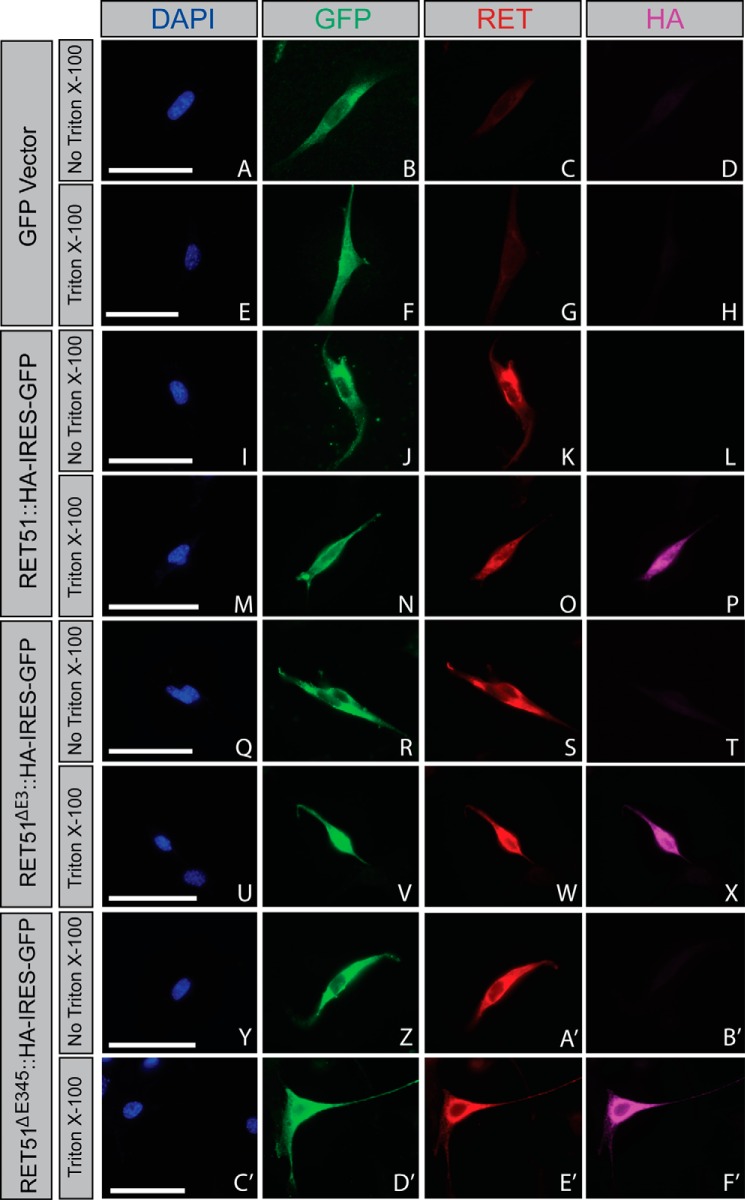
FIGURE 3.
RETΔE3 and RETΔE345 are trafficked to the cell surface. NIH/3T3 cells were transfected with GFP (A–H), Ret51::HA (I–P), Ret51ΔE3::HA (Q–X), or Ret51ΔE345::HA (Y–F'). Constructs for the RET51 isoforms were bicistronic, allowing for expression of GFP as an indicator for positive transfection, which was encoded by an IRES-GFP following RET cDNA sequences. Cells were washed in 1× PBS and fixed briefly in 4% paraformaldehyde 24 h post-transfection. Cells were then blocked in immunofluorescence blocking solution with or without Triton X-100 to allow for cell membrane permeabilization. In the absence of Triton X-100, antibodies specific for the RET extracellular domain were able to bind to RET51::HA (K), RET51ΔE3::HA (S), and RET51ΔE345::HA (A'). However, using an antibody against the HA epitope that would detect the C terminus located intracellularly for RET51::HA (L), RET51ΔE3::HA (T), and RET51ΔE345::HA (B') showed no binding/fluorescence in the absence of Triton X-100. When immunofluorescence blocking solution with Triton X-100 was used, fluorescence was detected using the anti-HA antibody for RET51::HA (P), RET51ΔE3::HA (X), and RET51ΔE345::HA (F'). These data show that RET51ΔE3 and RET51ΔE345 are trafficked to the cell surface similarly as full-length RET51. Scale bars represent 20 μm.