FIGURE 3.
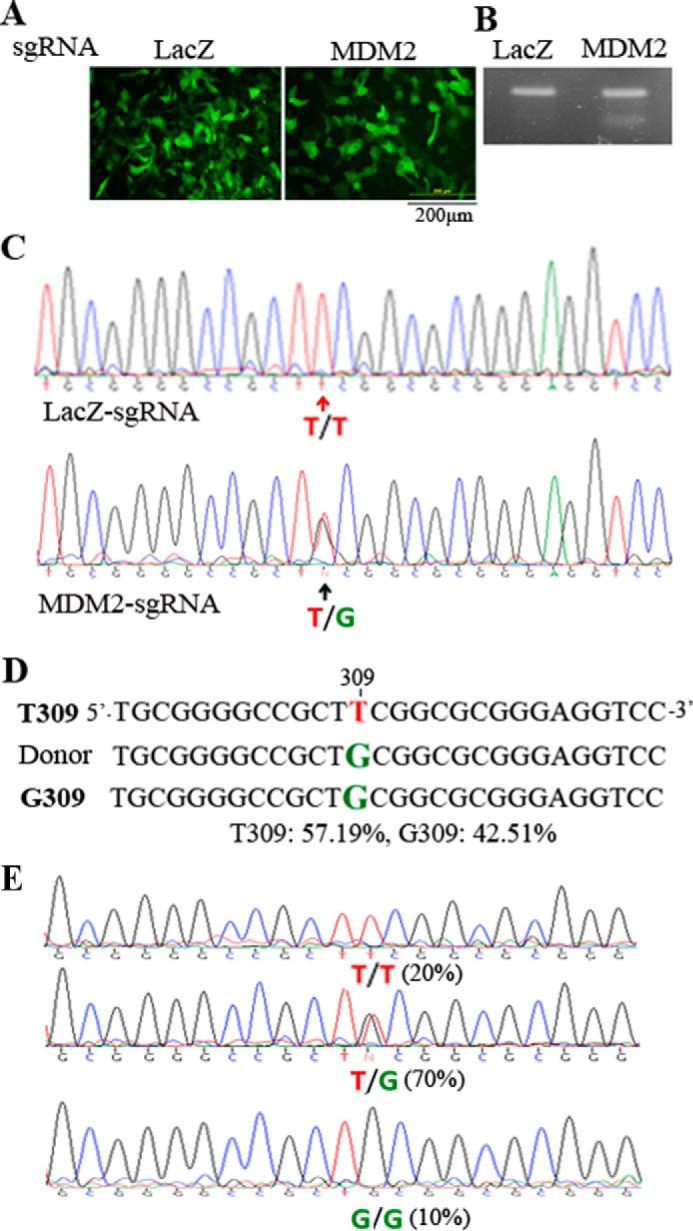
Creation of the genomic MDM2 T309G mutation. A, transfection of hPRPEs with dual AAV vectors and an ssHRT. The dual vectors of pAAV-SpCas9D10A plus pAAV-MDM2-sgRNA1 and 2 or pAAV-LacZ-sgRNA and the ssHRT were transfected into hPRPE cells by electroporation as described under “Experimental Procedures.” Subsequently, the cells were treated with Scr7 (5 μm). After transfection for 16 h, the cells were photographed under a fluorescence microscope. Scale bar = 200 μm. This is representative of three independent experiments. B, genomic DNA extracted from the transfected cells was used to amplify the region containing MDM2 SNP 309. The gel-purified PCR products were subjected to a Surveyor nuclease assay. This is representative of two independent experiments. C, the purified PCR products in B were also subjected to Sanger DNA sequencing. The top and bottom panels were from hPRPE cells transfected with LacZ-sgRNA and MDM2-sgRNA1 and 2, respectively. D, the purified PCR products from B were also analyzed by next-generation sequencing. The results showed that there was 57.19% MDM2 T309 and 42.51% MDM2 G309 in transduced cells with SpCas9D10A plus MDM2-sgRNA1 and 2 and ssHRT. E, single cells from transduced cells (B) were sorted by FACS into PCR tubes containing 5 μl of QuickExtract DNA extraction buffer, and the genomic DNA was isolated for PCR amplification of DNA fragments around the SNP. The purified DNA fragments from the 10 single cells were subjected to Sanger DNA sequencing. There were two 309T/T cells (20%), seven 309T/G cells (70%), and one 309G/G cell (10%) in the 10 sorted single cells.
