VOLUME 291 (2016) PAGES 8686–8700
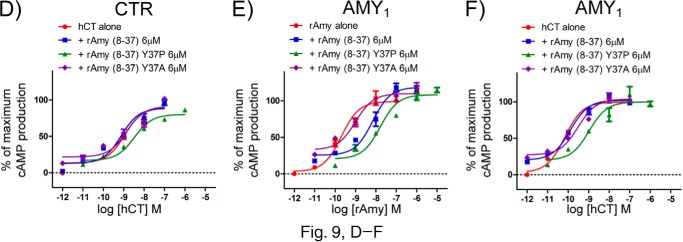
The rAmy(8–37) Y37A and Y37P peptides used in this study were synthesized with an additional Tyr at their N termini to permit quantification by UV absorbance. Due to an oversight, we did not detail this in the original publication and compared these mutant peptides with the WT rAmy(8–37) lacking an N-terminal Tyr. We have now repeated the relevant experiments with rAmy(8–37) Y37A and Y37P peptides lacking the N-terminal Tyr addition. Concentrations of the peptides were determined by assuming 80% peptide content for the lyophilized powder. The ECD binding results in Figs. 6B and 8B were unchanged (data not shown). Unfortunately, the cell signaling assays in Fig. 9, D–F, were affected by this error, likely because a Tyr at the N terminus of rAmy(8–37) contacts the CTR 7-TM domain. The corrected Fig. 9 panels and the corresponding Table 3 pA2 values provided below indicate that the addition of an N-terminal Tyr masked a defect of the Y37A alteration and caused the Y37P mutant to appear more potent than it is. Although our original statement that rAmy Tyr-37 is dispensable for receptor binding is incorrect, the true rAmy(8–37) Y37P peptide retained selectivity for AMY1 over CTR, and thus our proposed allosteric mechanism for RAMP alteration of CTR selectivity is still supported by the data. The overall conclusions of the paper were not affected. We apologize for any inconvenience this error may have caused.
TABLE 3.
pA2 of antagonists for cAMP production mediated by CTR and AMY1 receptors
Analysis of variance with Tukey's post hoc test was used for statistical analysis.
| Peptide | CTR with hCT agonist |
AMY1 with rAmy agonist |
AMY1 with hCT agonist |
||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean | S.E. | n | Mean | S.E. | n | Mean | S.E. | |
| rAmy(8–37) | 5 | <5.2 | 7 | 5.96 | 0.07 | 7 | <5.4 | ||
| rAmy(8–37) Y37P | 3 | 5.96 | 0.19 | 4 | 6.77b | 0.16 | 4 | 6.12 | 0.11 |
| rAmy(8–37) Y37A | 3 | <5.2 | 4 | <5.4 | 4 | <5.4 | |||
b p < 0.05 compared with rAmy(8–37).