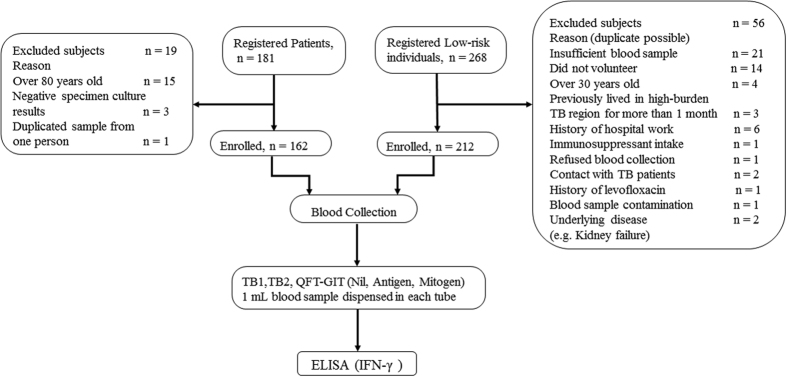
Figure 1. Flow diagram and study design.
The reasons for exclusion of the registered patients and low-risk individuals are shown in Fig. 1. A 1 mL blood sample collected from enrolled TB patients and low-risk individuals was dispensed into each tube (TB1 and TB2 tubes of QFT-Plus assay, and nil, mitogen, antigen tubes of QFT-GIT assay). After incubation and centrifugation, the supernatant plasma of each tube was used to perform ELISA and to obtain the concentration of released IFN-γ. QFT-GIT: QuantiFERON-TB Gold In-Tube; ELISA: enzyme linked immunosorbent assay; TB1, TB2: Antigen tubes of QuantiFERON-TB Gold Plus.