Abstract
Objectives
The prevalence of depression is high in patients with Crohn’s Disease (CD). We examined the influence of affective-cognitive symptoms of depression on the risk of exacerbation of CD.
Methods
We studied 2144 adult volunteers with a self-reported diagnosis of CD who completed a baseline survey that included demographics, CD status and an affective-cognitive index of depression. Linear and logistic regression analyses were used to determine if CD status at 12 months was associated with the baseline measure of depression. Analyses were adjusted for confounders including age, gender, race, baseline disease activity, disease duration, prior hospitalization and surgery, corticosteroid and anti-TNF use, medication adherence, BMI, current smoking, education, and sleep quality.
Results
Depression was significantly associated with subsequent increases in SCDAI score in both unadjusted (p<0.001) and adjusted (p< 0.001) analyses. This association was non-linear, with a shallower slope for lower levels of depression. A 10 point increase in depression t-scores from 55 to 65 was associated with a 18.6 point increase in SCDAI (95% CI 11.5–25.6) and an odds ratio of 1.27 for SCDAI > 150 at follow up (CI: 1.01–1.60). We also found a significant association between depressive symptoms and hospitalization.
Conclusion
Cognitive-affective depressive symptoms were significantly associated with a risk of exacerbation of CD and hospitalization.
INTRODUCTION
Crohn’s disease (CD) is a chronic bowel condition affecting more than 700,000 people in North America (1). CD is characterized by episodes of exacerbations and remissions. Because the course is unpredictable and there is no cure, disease management must focus on achieving and maintaining remission, preventing complications, improving quality of life and minimizing the impact of comorbid conditions.
The rates of depression among CD patients appear to be higher (2) than the general population. The key question is whether depression influences the manifestation and severity of CD or if depression is a consequence of suffering from a debilitating chronic disease. Therefore, the aim of this study was to determine whether there is a relationship between depressive symptoms and subsequent CD activity.
Earlier cohort studies reporting comorbidity of CD and patients’ mood used depression measures that include physical symptoms that overlap with those seen in CD such as disordered sleep, fatigue and poor appetite (3, 4). To eliminate the overlap and avoid the possibility of spurious relationships between measures of exposure and outcome, this study restricts the ascertainment of depression to one dimension of depression, affective-cognitive symptoms. It is yet unclear which symptom dimensions of depression are associated with CD activity and this will be the first study with adult CD patients of the relationship between a specific symptom dimension of depression and later CD activity. Different subsets of depressive symptoms have been investigated with adolescents with IBD (5). The principal hypothesis for this study is: CD patients experiencing affective-cognitive symptoms of depression at baseline were more likely to report active CD 12 months later. A secondary hypothesis is that CD patients experiencing affective-cognitive symptoms of depression at baseline will have more frequent hospitalizations, surgeries, and anti-TNF (anti- Tumor Necrosis Factor) usage to treat their CD because of a more severe or persistently active CD.
METHODS
Study Population
Subjects were volunteers in CCFA Partners, an internet-based cohort study sponsored by the Crohn’s and Colitis Foundation of America (CCFA). This cohort has been described in detail in previous publications (6). In brief, CCFA Partners is a longitudinal Internet-based cohort of volunteers with self- reported inflammatory bowel disease. Participants had been invited to participate in the cohort through the CCFA e-mail roster, social media, and at educational events. During the first recruitment volunteers were excluded if they were under age 18 and those who did not have self-reported IBD (CD, UC, or indeterminate colitis). Volunteers also had to have Internet access to join the e-cohort and complete the surveys. There were no other exclusion criteria for participation. All volunteers completed a baseline survey comprising demographics and information about their IBD. Optional modules on various patient reported outcomes were included with the baseline survey. Volunteers were invited to complete a follow-up questionnaire every 6 months to report disease activity, changes in the treatment and repeated measures of patient reported outcomes. The study protocol was approved by the Institutional Review Board at the University of North Carolina, Chapel Hill, North Carolina and Vanderbilt University, Nashville TN.
Instruments
For this study, all volunteers completed a depression symptom questionnaire (PROMIS) (7) that measured exposure and a CD symptom questionnaire (SCDAI) (8) at baseline and 12 months later that measured outcome.
Depression was measured by using the National Institutes of Health Patient Reported Outcomes Measurement Information Systems (PROMIS) depression 4-item short form (7). In validation studies the PROMIS depression test bank items correlated with the Center for Epidemiological Studies Depression Scale, r= 0.83. The PROMIS depression questionnaire used in this study did not include items assessing somatic symptoms that overlap with symptoms of CD such as poor sleep, fatigue and lack of appetite. The short form was composed of four Likert-type five point items measuring the frequency of negative mood (I felt depressed) and cognition: (negative beliefs about the self (I felt worthless, I felt helpless), decreased life engagement/negativity to the future (I felt hopeless) during the previous seven days. It was administered at baseline and follow up. All responses were summed to form a raw score ranging from 4 to 20, which was then converted to a standardized score (t-score). For all PROMIS measures the t-score for the standardizing population is 50 with a standard deviation equal to 10. Thus, a t-score change from 55 to 65 represents a change from 0.5 standard deviations to 1.5 standard deviations above the standardizing population mean.
Disease activity at baseline and the 12-month follow up was assessed by the Short Crohn’s Disease Activity Index (SCDAI) (8). Disease activity was modeled as both a continuous SCDAI t-score and a binary outcome with active disease being defined as a SCDAI t-score greater than 150. The SCDAI was developed to provide a shortened and simplified Crohn’s Disease Activity Index (CDAI) using a patient’s self-report. We considered any abdominal surgeries within 12 month, any hospitalizations within 12 months, and use of anti-TNFs at 12 months as secondary outcomes because these medical interventions are often associated with greater CD activity.
Covariates
Based on the research literature, the following variables were included in the regression models as potential confounders of the association between depression and CD activity: age, gender, race, baseline SCDAI, disease duration, anti-TNF use, BMI, current smoking, level of educational attainment, and sleep (9). For the anti-TNF outcome, we also adjusted for baseline anti-TNF usage. Post-hoc analyses were conducted where we additionally adjusted for baseline history of hospitalization/surgery, use of steroids, and adherence to oral medications (10) to confirm our findings.
Statistical Analysis
Continuous variables were summarized using the median, 25th, and 75th percentile, and categorical variables were summarized using percentages in each category. The Kruskal-Wallis test (continuous variables) and Pearson’s chi-square tests (categorical variables) were used to compare baseline covariates by depression t-score quartile.
In this prospective cohort study we represented baseline disease activity as continuous instead of as a dichotomized variable – active versus inactive - as advocated by methodologists (11, 12). Use of baseline disease activity as a continuous variable allowed us to operate without any assumptions about the shape of the relationship, use all the information about disease activity at hand and allowed us to detect any non- linearity in the relation between exposure (depressive symptoms) and outcome (CD activity).
Linear regression was used to model continuous outcomes and logistic regression was used to model binary outcomes. From the linear regression models, we report the adjusted expected change in mean SCDAI at 12 months per 10 point increase in baseline depression. From the logistic regression models, we report the odds ratio for having active disease (defined as SCDAI >150) per 10 point increase in baseline depression t-score. We pre-specified that depression would be flexibly modeled as a continuous predictor while making minimal assumptions about the form of the relationship between depression symptoms and the outcome. This was accomplished by modeling depression using a restricted spline (3 d.f.).
Based on the results of the linear regression analysis of SCDAI, we found that the slope for the depression t-score changed at 55, being flatter below 55 and steeper above 55. We report our results from all regression models in reference to 10-point changes in depression t-score using intervals of 45 to 55, 55 to 65, and 65 to 75. Statistical significance of the depression construct was assessed using 2 d.f. Wald tests to simultaneously test each of the depression coefficients included in the regression model.
Sensitivity analyses were also conducted where we treated depression t-score as a continuous variable, but without using restricted cubic splines to flexibly model the association. Logistic models were estimated with the primary and secondary outcomes. For these models, we report the unadjusted and adjusted association of a 10-point increase in depression t-score with each outcome. Confounding was evaluated by looking for a 10% change in the estimated regression coefficient for depression t-score.
RESULTS
A total of 5707 subjects with CD completed baseline surveys, 2144 of whom also completed a 12-month follow up SCDAI. Volunteers without 12 month follow up surveys were younger (median age 40 versus 43 years), less likely to be white (92% versus 95%), more likely to be current smokers (12% versus 7%), less educated (65% college and above versus 73%) and likely to be more depressed (median t-score 52.7 +/− 10.0 versus 51.4 +/− 9.2). There was no evidence that duration of diagnosis, sex, BMI, or sleep scores differed between analyzed (N=2144) and lost to follow (N=3775) subjects. Table 1 shows the distribution of patient characteristics by extent above or below a depression t-score score of 50. Respondents with the most severe depressive symptoms at baseline were younger, more recently diagnosed with CD, were smokers, were heavier and reported lower levels of educational attainment.
Table 1.
Patient demographics for included subjects by baseline depression t-score (N=2144). Continuous variables are summarized by the median and [25th, 75th] percentiles (IQR) and tested with the Kruskal-Wallis test. Categorical variables are tested with Pearson’s chi-squared test.
| Depression t-score | 41 or less (n=735) | 41 to 50 (n=294) | 50 to 60 (n=696) | 60 or more (n=419) | P-value |
|---|---|---|---|---|---|
| Age at baseline, years [IQR] | 45 [32, 58] | 42 [32, 55] | 43 [30, 54] | 42 [29, 53] | 0.002 |
| Age at IBD diagnosis [IQR] | 25 [19, 35] | 26 [19, 37] | 25 [18,37] | 25 [19, 35] | 0.73 |
| Length of diagnosis [IQR] | 13 [6, 25] | 11 [4, 20] | 11 [5, 23] | 10 [4, 20] | <0.001 |
| BMI, kg/m2 [IQR] | 24 [21, 27] | 24 [21, 27] | 25 [22, 29] | 25 [22, 30] | <0.001 |
| Sleep t-score | 52 [51, 54] | 52 [51, 54] | 52 [51, 54] | 52 [51, 54] | 0.004 |
| Current smoker [n] | 4% [31] | 4% [13] | 7% [49] | 14% [57] | <0.001 |
| Male sex, % | 28% | 27% | 25% | 20% | 0.02 |
| White race, % | 96% | 94% | 95% | 94% | 0.37 |
| Education level, % | <0.001 | ||||
| Less than high school | 1% | 0% | 0% | 1% | |
| Graduate high school | 6% | 4% | 7% | 7% | |
| Some college | 16% | 18% | 22% | 29% | |
| College graduate | 42% | 39% | 43% | 42% | |
| Graduate school | 36% | 38% | 28% | 20% |
Association between baseline depression and 12-month SCDAI and secondary outcomes
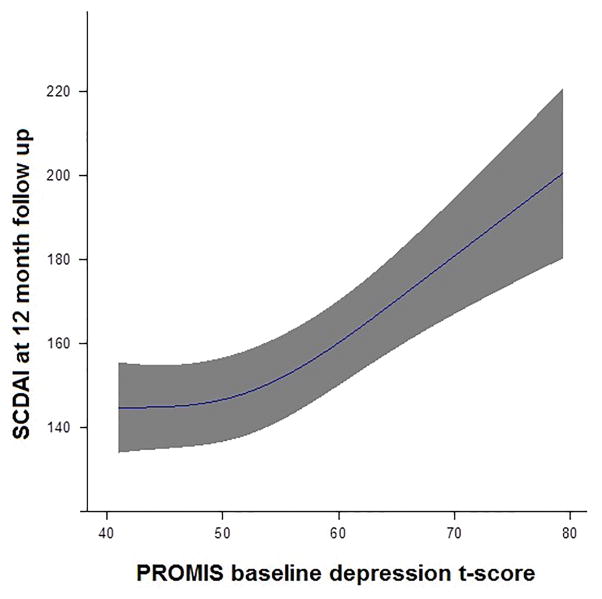
We found a significant association between depression t-score and subsequent SCDAI score, controlling for baseline differences in SCDAI score, in both unadjusted (p<0.001) and adjusted (p<0.001) linear regression models. Furthermore, we found this relationship to be curved with a shallower slope below 55 and a steeper slope above 55. Figure 1 depicts this association by plotting the expected SCDAI versus depression t-score while adjusting for other covariates by fixing continuous covariates at their mean and categorical covariates at their mode. Adjusting for covariates, a 10 point increase in depression t-score from 55 to 65 was associated with a 18.6 point increase in SCDAI (95% CI: 11.5 to 25.6). A 10 point increase in depression t-score from 45 to 55 was only associated with a 6.9 point increase (95% CI: 2.4 to 11.4) in SCDAI.
Figure 1.
Association between baseline depression t-score and SCDAI at 12 month follow up with 95% confidence interval. Depression t-score was modeled flexibily using restricted cubic splines to allow for a curved relationship. SCDAI is estimated from a multivariable model and represents an average subject with a baseline SCDAI of 150, sleep t-score of 50, length of diagnosis 15 years, white race, non-current smoker, 42 years old, BMI of 26, and college education.
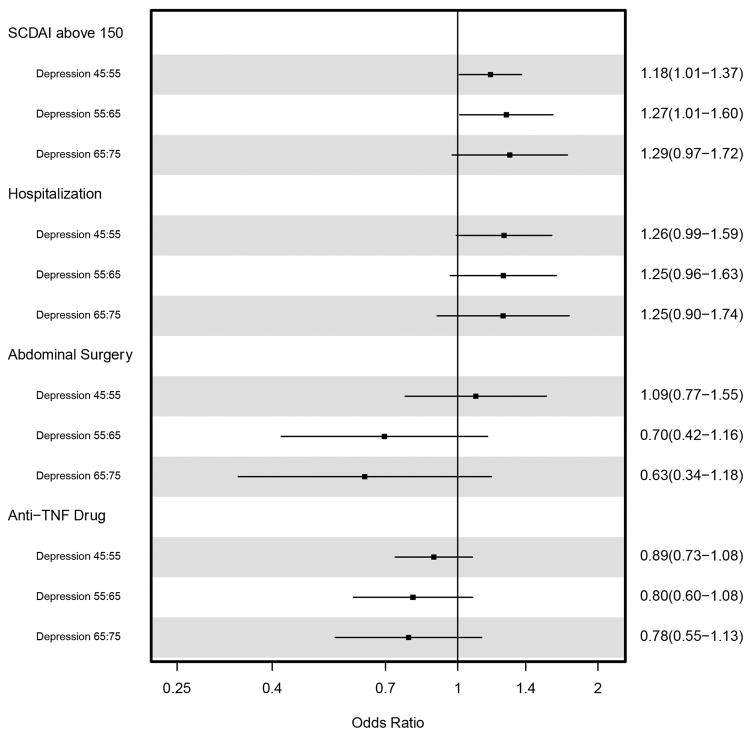
There was also a significant association between elevated depression t-score and the odds of reporting active CD 12 months later in both unadjusted and adjusted logistic regression models. Figure 2 summarizes the odds ratios from the adjusted models for 10 point increases in baseline depression t-score for the primary and secondary outcomes. Because we used regression splines to flexibly model the association between depression t-score and each outcome, a 10-point increase in depression t-score was not constrained to be the same for all 10-point increases. In Figure 2, we present the odds ratios associated with changes from 45 to 55, 55 to 65, and 65 to 75 in t-score. For SCDAI > 150, the odds ratios for a change from 55 to 65 and 65 to 75 (OR=1.27,95% CI 1.01–1.60, and OR=1.29, CI 95% 0.97–1.72, respectively) were larger than the odds ratios for a change from 45 to 55 (OR=1.18, 95% CI 1.01–1.37) in depression t-score. Overall, there was a significant association between baseline depression t-score and the odds of active CD (p=0.001, df=2). Hospitalizations were also significantly associated with depression t-score in adjusted models (p=0.03, df=2) and the odds ratios were similar for increases from 45 to 55, 55 to 65, and 65 to 75. There was no evidence that abdominal surgeries (p=0.34, df=2) or anti-TNF drugs (p=0.15, df=2) were associated with depression t-score when depression was modeled flexibly using splines.
Figure 2.
Adjusted fold increase in the odds of SCDAI above 150, hospitalizations, abdominal surgery, and anti-TNF usage per 10 point increase in depression t-score. Depression t-score was flexibly modeled using restricted cubic splines to allow for different odds ratio for a 45 to 55, 55 to 65, and 65 to 75 point increase in t-score.
Supplemental Analyses
Supplemental analyses were conducted to further scrutinize the conclusions from our primary model. For these analyses we treated depression t - scores as a continuous variable, but without using restricted cubic splines to flexibly model the association. Logistic models were estimated for the primary and secondary outcomes, and we computed the unadjusted and adjusted associations of a 10-point increase in depression t - score with each outcome. We also conducted an additional analysis where we adjusted for medication adherence scores, baseline history of hospitalization/surgery, use of steroids, and our other previously included confounders. In the 1285 participants for whom the medication adherence score was available, we observed similar, albeit slightly stronger association between baseline depression and disease activity at 12 months. There was also no evidence of residual confounding based upon our approach utilizing a 10% change in the estimated regression coefficient for depression t - score.
DISCUSSION
Depression is a common comorbidity for patients with CD (2). Depression impacts on the quality of patients’ lives (13), cost of treatment (14), adherence to disease management (15) and may even alter the biologic potential for controlling the status of the disease (16). Hence, we hypothesized that comorbid depressive symptoms in adults with CD would predict higher levels of CD activity a year later. We tested this hypothesis prospectively using validated measures of depressive symptoms (exposure) and CD activity (outcome). Controlling for confounding variables, the CD volunteers who reported higher levels of depressive symptoms had a greater odds of reporting more frequent diarrhea, pain and reduced well-being 12 months later than less depressed CD volunteers. Further, CD volunteers with more severe depressive symptoms subsequently reported higher odds of hospitalization but not surgery or anti-TNF usage.
Our study supports the findings of earlier cohort studies showing that depression is associated with subsequent CD activity (3, 4, 17), suggesting a temporal relationship between depression and subsequent CD activity. However, what is unique about this study is the finding that the association between depression and subsequent CD activity is stronger when there are higher levels of baseline depressive symptoms, suggesting a dose response relationship. Heightened depression, especially the cognitive characteristic of negativity to the self and the future as studied in this study, maybe a psychological condition that influences CD morbidity through physiological processes and indirectly via behavioral pathways. The health risk behaviors and psychobiological changes associated with depression increase the risk for chronic medical disorders, and biological changes and complications associated with chronic medical disorders may precipitate depressive episodes (18). For example, the CD volunteers who reported greatest negativity to the self and the future and sadness at baseline were also more likely to be smokers who were overweight and less educated. Controlling for these factors attenuated the effect estimates but they remained significant. More needs to be known about depressed mood and negativity toward the self and the future in terms of associated eating patterns, medication use and adherence and physical activity in order to understand how the affective-cognitive dimension of depression influences health risk behavior and the immune system of depressed CD volunteers. Depression and its symptom dimensions are now being investigated in depressed chronic disease patients with myocardial infarction (19) and diabetes (20). For example, study of the effects of depressive symptoms on immunological parameters in heart failure patients (21) showed that the level of cognitive/affective depression at baseline was positively associated with hsCRP (high sensitivity C Reactive Protein), sTNFR1 and sTNFR2 (Tumor Necrosis Factor soluble receptors) at 12-month follow up, while somatic/affective depression at baseline was positively associated with sTNFR1 and sTNFR2.
While there is consistency in the findings across the limited number of cohort studies of CD and comorbid depression, the previous studies were conducted under very different conditions. Our study had methodological advantages from prior investigations. Our cohort study was not limited to a tertiary referral center and is the first to our knowledge to eliminate the influence of overlapping somatic symptoms of depression and CD on the results. In previous cohort studies of comorbid CD and depression, depression was treated as a homogenous construct without regard to the varied symptoms of depression (3, 4, 17). It is possible that these earlier cohort studies of depression and CD may have demonstrated correlation because the somatic symptoms of depression may be more strongly linked to the mechanisms thought to underlie the depression-CD relationship (22). Another advantage of the present study was the use of CD activity level as a continuous variable at baseline instead of the more typical dichotomization which results in reduced power. This allowed for the detection of possible non-monotonic relationships between symptoms of depression and CD activity level. Our findings, therefore, provide more definitive data demonstrating that higher levels of affective-cognitive symptoms of depression are associated with subsequent increased disease activity and need for hospitalization even after controlling for known confounders.
There are limitations that need to be acknowledged. First, we have explored the relationship of depressive symptoms and CD in a large internet based cohort of CD patients. The sampling introduced volunteer self-selection, resulting in a cohort composed largely of Caucasian women who took fewer health risks and were less depressed and reported less active disease at the baseline. The study thus may under-represent individuals who might show the hypothesized relationship. Although the prospective study design with appropriate control for a number of potential confounders provides a high degree of internal validity, attrition was extremely high in this study with a loss to follow up of 62%. Analysis of the differences between those CD volunteers who completed the 12 month follow up SCDAI and those who did not demonstrated that the non-responders had higher t-score on the depression measure. However, this difference in mean t-scores was only 1.3 which, while significant, is below the threshold of a minimal important difference for this measure. In addition, in using the four item PROMIS abbreviated depression scale we did not allow a direct comparison of different symptom dimensions of depression including affective-cognitive and affective-somatic symptoms in the prediction of subsequent CD activity level. Although we do not know if the CD volunteers had been treated with antidepressant medication or psychotherapy, in reality, that information is not relevant to the association between affective-cognitive depressive symptoms and the SCDAI. Finally, The SCDAI is a patient reported measure of disease symptoms, rather than a direct measure of intestinal inflammation. Hence, we cannot fully evaluate a direct causal relationship between symptoms of depression and increased mucosal inflammation. As such, we encourage future research on comorbid depression and CD activity using measures of inflammation to help elucidate the full impact of depressive symptoms on inflammatory processes. However, given recently increasing interest in patient-reported outcomes research, our results contribute to improved understanding of the role of depression in predicting which patients maybe more likely to experience symptoms of increased CD activity.
Although use of the study’s longitudinal design suggests that affective-cognitive symptoms of depression are antecedent to CD activity twelve months later, both CD and depressive symptoms have potentially long induction periods making it difficult to establish the precise period “at risk”. This raises possible concern about reverse causality, with earlier CD activity influencing the incidence of depressive symptoms rather than the converse. In order to clarify the sequencing of depressive symptoms and CD activity and understand their dynamic interaction, longitudinal panel data will be helpful. Measures of depression (exposure) must include both cognitive and somatic symptoms of depression and their interaction over time. Crohn’s disease activity (outcome) must be measured by objective methods, such as fecal calprotectin, and from patients’ symptom appraisals, and for longer periods and with shorter exposure- outcome intervals before coming to valid conclusions about the temporal relationship between CD activity and symptom dimensions of depression.
The present study expands and informs the field in two very important ways. The first is that it suggests that CD patients who also have affective-cognitive symptoms of depression are more likely to have more severe CD symptoms later in time. The second is that it starts to chart the map for future research about comorbid depression and CD, starting with the examination of possible reverse causality, the assessment of measurement invariance, and possible health behavior and immunological mediators of the relationship of depressive symptoms and CD activity. In the present time, however, even without complete understanding of causality or mechanism, this observation about comorbid depression and subsequent CD activity and hospitalizations makes a strong case for routine assessment of depression when evaluating and treating CD. Depressive symptoms comorbid with CD seem to add to the cost of treatment and are associated with lower quality of life and poorer clinical outcomes. Management of depression should be integral to the management of CD patients.
Negativity to the self and the future and depressed affect as measured here may represent adaptational difficulties which could influence self-management, the immune system and ultimately disease progression (20). Given the substantial challenges that result from exacerbations of CD, gastroenterologists need to be alert to depression in CD patients and refer for appropriate management.
Table 2.
Unadjusted and adjusted fold increase in the odds of SCDAI above 150, hospitalization, abdominal surgery, and anti-TNF usage per 10 point increase in depression t-score. Depression t-score was modeled using as a single linear term.
| Outcome | Unadjusted Odds Ratio [95% CI] | Adjusted Odds Ratio |
|---|---|---|
| SCDAI above 150 | 1.87 [1.69, 2.08] | 1.21 [1.07, 1.36] |
| Hospitalization | 1.66 [1.42, 1.94] | 1.26 [1.06, 1.49] |
| Abdominal surgery | 1.07 [0.81, 1.41] | 0.94 [0.72, 1.22] |
| Anti-TNF usage | 1.04 [0.95, 1.14] | 0.91 [0.78, 1.08] |
Study Highlights.
What is current knowledge?
Rates of depression among CD patients is higher than the general population
Clinic-based cohort studies show that depression predicts subsequent CD activity
Prior studies of CD and depression assume all depression symptoms are roughly equivalent
What is new here?
Affective-cognitive symptoms of depression predicted subsequent CD activity and hospitalization rate, suggesting a temporal relationship
CD activity is greater with baseline higher levels of affective-cognitive symptoms, suggesting a dose-response relationship
Symptom dimensions of depression maybe important in understanding the association of depression and self-reported CD activity
Acknowledgments
Drs. Stephen Entman and Robert Levine were helpful in discussing the data and in preparing the manuscript and Tara Duffie in the submittal process.
Funding/support and role of sponsor: This research was supported, in part, by awards from the National Center for Advancing Translational Science/National Institute of Health (NO. UL1TR000445), Crohn’s and Colitis Foundation of America and the National Institutes of Health (P30 DK034967). The CCFA, National Center for Advancing Translational Science and the NIH had no role in the design, analysis and interpretation of the data, or the preparation, review, or approval of the manuscript.
Footnotes
Guarantor of the article: Gaines
Author Contributions: Drs. Gaines and Slaughter had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Gaines
Acquisition of data: Sandler, Long, Kappelman, Martin
Analysis and interpretation of the data: all authors
Drafting of the manuscript: Gaines
Critical revision of the manuscript for important intellectual content: all authors
Statistical analysis: Slaughter, Wang
Conflict of interest: There are no conflicts of interest to disclose for all named authors
References
- 1.Loftus EV, Schoenfled P, Sandborn WJ. The epidemiology and natural history of Crohn’s disease in population-based patient cohorts from North America: a systematic review. Aliment Pharmacol Ther. 2002;16:51–60. doi: 10.1046/j.1365-2036.2002.01140.x. [DOI] [PubMed] [Google Scholar]
- 2.Walker JR, Eidger JP, Graff LA, et al. The Manitoba IBD cohort study: a population –based study of the prevalence of lifetime and 12-month anxiety and mood disorders. Am J Gastroenterol. 2008;103:1989–97. doi: 10.1111/j.1572-0241.2008.01980.x. [DOI] [PubMed] [Google Scholar]
- 3.Mittermaier C, Dejaco C, Waldhoer T, et al. Impact of depressive mood on relapse in patients with inflammatory bowel disease: a prospective 18-month follow up study. Psychosom Med. 2004;66:79–84. doi: 10.1097/01.psy.0000106907.24881.f2. [DOI] [PubMed] [Google Scholar]
- 4.Mardini HE, Kip KE, Wilson JW. Crohn’s disease: a two year prospective study of the association between psychological distress and disease activity. Dig Dis Sci. 2004;49:492–7. doi: 10.1023/b:ddas.0000020509.23162.cc. [DOI] [PubMed] [Google Scholar]
- 5.Szigethy EM, Youk AO, Benhavon D, et al. Depression subtypes in pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2014;58:574–581. doi: 10.1097/MPG.0000000000000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Long MD, Kappelman MD, Martin CF, et al. Development of an internet-based cohort of patients with inflammatory bowel diseases (CCFAPartners): methodology and initial results. Inflamm Bowel Dis. 2012;18:2099–106. doi: 10.1002/ibd.22895. [DOI] [PubMed] [Google Scholar]
- 7.Pilkonis PA, Seung WC, Reise SP, et al. Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS): Depression, Anxiety, and Anger. Assessment. 2011;18:263–283. doi: 10.1177/1073191111411667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thia K, Faubion WA, Loftus EV, et al. Short CD AI: Development and validation of a shortened and simplified Crohn’s disease activity index. Inflamm Bowel Dis. 2011;17:105–11. doi: 10.1002/ibd.21400. [DOI] [PubMed] [Google Scholar]
- 9.Loftus EV. Epidemiology of Inflammatory Bowel Disease. In: Talley NK, Locke GR, Moayyed P, West J, Saito YA, editors. GI Epidemiology: Diseases and Clinical Methodology. 2. Chicester, West Sussex: John Wiley; 2014. pp. 273–284. [Google Scholar]
- 10.Morisky DD, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1987;24:67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Harrell FE, Slaughter JC. Information Loss. Introduction to Biostatistics for Biomedical Research. 2015;Chapter 17 Biostat.mc.vanderbilt.edu/ClinStat. [Google Scholar]
- 12.Royston P, Altman DG, Sauerbrei W. Dicohotomizing continuous predictors in multiple regression: a bad idea. Statistics in Medicine. 2006;25:127–141. doi: 10.1002/sim.2331. [DOI] [PubMed] [Google Scholar]
- 13.Zhang CK, Hewett J, Hemming J, et al. The influence of depression on quality of life in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:1732–9. doi: 10.1097/MIB.0b013e318281f395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ray I, Stiles R, Domenico H, et al. Cost of outpatient treatment of Crohn’s disease with comorbid depression in an academic medical center. Digestive Disease Week; Orlando, Fla. May 2013.2013. [Google Scholar]
- 15.Shale MJ, Riley A. Studies of compliance with delayed-release mesalazine therapy in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2003;18:191–198. doi: 10.1046/j.1365-2036.2003.01648.x. [DOI] [PubMed] [Google Scholar]
- 16.Persoons P, Vermeire S, Demyttenaere K, et al. The impact of major depressive disorder on the short- and long-term outcome of Crohn’s disease treatment with infliximab. Aliment Pharmacol Ther. 2005;22:101–10. doi: 10.1111/j.1365-2036.2005.02535.x. [DOI] [PubMed] [Google Scholar]
- 17.Ananthakrishanan AN, Khalili H, Pan A, et al. Association between depressive symptoms and incidence of Crohn’s disease and ulcerative colitis: results from the Nurses’ Health Study. Clin Gastroenterol Hepatol. 2013;11:57–62. doi: 10.1016/j.cgh.2012.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katon WJ. Epidemiology and treatment of depression in patients with chronic medical illness. Dialogues Clin Neurosci. 2011;13:7–23. doi: 10.31887/DCNS.2011.13.1/wkaton. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Groenewold NA, Doombos B, Zuidesma M, et al. Comparing cognitive and somatic symptoms of depression in myocardial infarction patients and depressed patients in primary and mental health care. PLosOne. 2013;8(1):e53859. doi: 10.1371/journal.pone.00589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiltink j, Michal M, wild PS, et al. Associations between depression and diabetes in the community: Do symptom dimensions matter? Results from the Gutenberg Health Study. PLoS One. 2014;9(8):e 105499. doi: 10.1371/journal.pone.0105499-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kupper N, Widdershoven JW, Pedersen SS. Cognitive/affective and somatic/affective symptom dimensions of depression are associated with current and future inflammation in heart failure patients. J Affective Disorder. 2012;136:567–576. doi: 10.1016/j.jad.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 22.Rampton D. The influence of stress on the development and severity of immune-mediated diseases. J Rheumatol Suppl. 2011;88:43–47. doi: 10.3899/jrheum.110904. [DOI] [PubMed] [Google Scholar]
- 23.Peyrin-Biroulet L, Reinisch W, Colombel JF, et al. Clinical disease activity, C-reactive protein normalization and mucosal healing in Crohn’s disease in the SONIC trial. Gut. 2014;63:88–95. doi: 10.1136/gutjnl-2013-304984. [DOI] [PubMed] [Google Scholar]