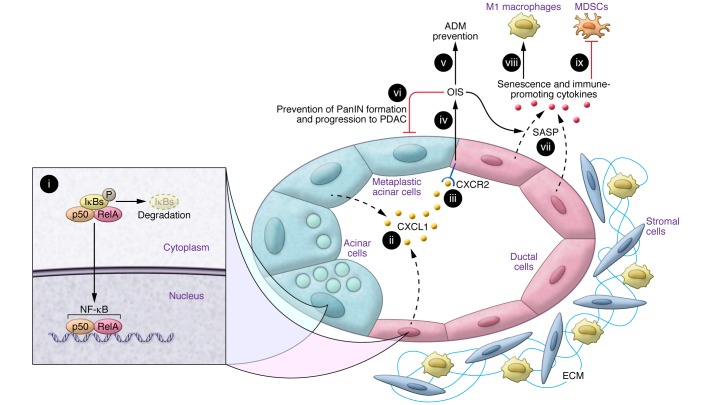
Figure 1. RelA exerts tumor-suppressive actions during the early steps of PDAC initiation.
An early pancreatic lesion, in which acinar cells are undergoing ADM and are actively forming a PanIN lesion. (i) Activated RelA translocates to the nucleus with its p50 partner, and the NF-κB complex enhances the production of CXCL1 (ii). After binding to CXCR2 (iii), CXCL1 enhances OIS (iii), which attenuates the formation of ADM lesions (v), as well as PanIN formation and progression to PDAC (vi). As a result of the induction of senescence, the ductal cells in PanIN lesions secrete multiple additional cytokines (vii) through a mechanism termed SASP. These RelA-induced changes are associated with paracrine immune–promoting actions on the tumor microenvironment, as evidenced by an increase in the number of M1 macrophages (viii) and a decrease in the number of myeloid-derived suppressor cells (MDSCs) (ix). Minimal stroma is shown for these early lesions. By contrast, in established PDAC, loss of p53 or p16/Rb pathways leads to senescence bypass, which converts RelA into a tumor promoter. ECM, extracellular matrix.