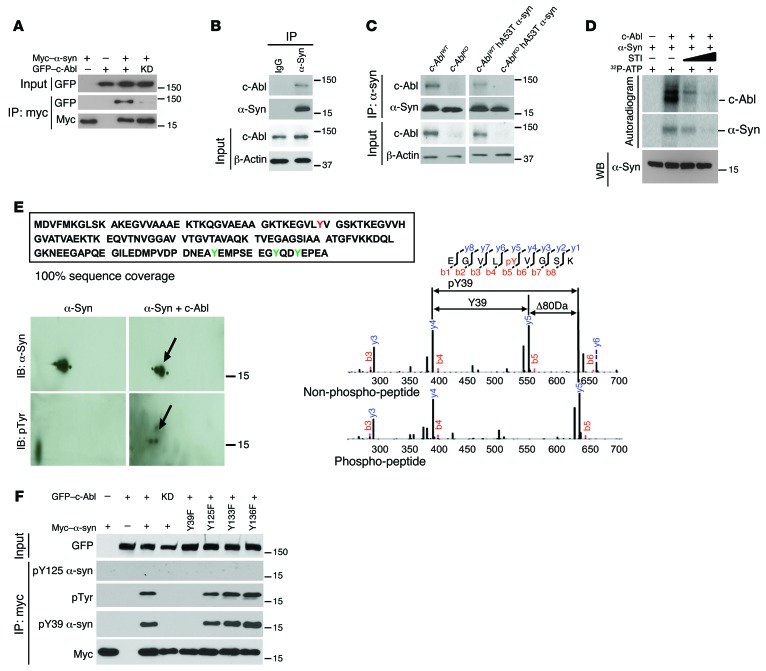
Figure 6. c-Abl interacts with and phosphorylates α-synuclein.
(A) Coimmunoprecipitation of myc-tagged α-syn (myc-α-syn) and GFP-tagged c-Abl (GFP-c-Abl) by anti-myc antibody in SH-SY5Y cells cotransfected with myc-α-syn and GFP-c-Abl or kinase-dead (KD) (lysine 290 arginine) version of c-Abl (GFP-c-Abl-KD) followed by IB. (B) Coimmunoprecipitation of α-syn and c-Abl by anti–α-syn antibody in the brain stem from nontransgenic mice followed by IB. Anti-IgG was used as a negative control. (C) Coimmunoprecipitation of α-syn and c-Abl by anti–α-syn antibody in the brain tissue lysates from WT, c-Abl knockout (c-AblKO), hA53Tα-syn, and c-AblKO hA53Tα-syn mice followed by IB. (D) In vitro kinase assay showing that c-Abl phosphorylates α-syn. Autoradiogram indicates that STI-571, a c-Abl kinase inhibitor, dose-dependently reduced phosphorylation of α-syn and c-Abl. Immunoblot in the bottom panel shows equivalent amount of α-syn used in the experiment. (E) c-Abl phosphorylates α-syn on tyrosine (Y) 39. Mass spectrometric analysis reveals 100% sequence coverage of α-syn, showing that all tyrosine residues were investigated for phosphorylation status. Phosphorylated Y39 is indicated in red; other tyrosines are indicated in green (top). α-Syn phosphorylated by c-Abl was separated by 2-DE followed by IB (bottom left). The arrows indicate tyrosine phosphorylation of α-syn. Both nonphosphorylated and phosphorylated α-syn were subjected to liquid chromatography–tandem mass spectrometry (LC-MS/MS) to identify the phosphorylation site (bottom right). LC-MS/MS spectra of the nonphosphorylated peptide (EGVLYVGSK) and the phosphorylated peptide (EGVLpYVGSK) are compared, demonstrating that there is the 80-Da shift for the Y39 ion containing the phosphate moiety. The phosphorylated amino acid is preceded by a “p” and highlighted in red. (F) IP of myc-α-syn by anti-myc antibody in SH-SY5Y cells cotransfected with indicated plasmids followed by IB with anti-myc, anti-pY39 α-syn, anti-pTyr, or anti-pY125 α-syn antibodies. Inputs were immunoblotted with anti-GFP antibodies. All experiments were repeated at least 3 times.