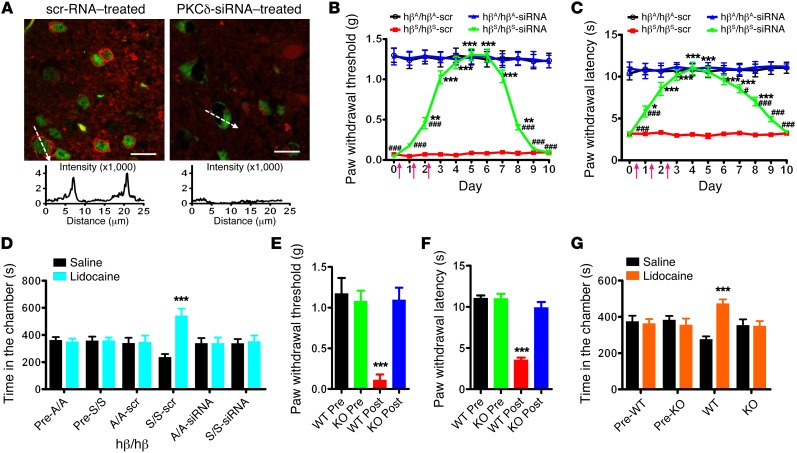
Figure 3. Sickle cell pain behaviors in mice after neuronal PKCδ silencing and in PKCδ-KO mice.
(A) Spinal delivery of RVG-9R/PKCδ-siRNA (2 μg/d for 3 days, i.t.) specifically knocked down PKCδ (red) in NeuN-positive (green) cells. Scale bars: 20 μm. n = 15 slices from 3 mice. PKCδ fluorescent intensity across representative cells (indicated by dashed arrows) is illustrated in the chart. Mechanical (B) and thermal (C) sensitivities were tested before and after siRNA treatments. Arrows denote siRNA injections. *P < 0.05, **P < 0.01, ***P < 0.001 vs. hβS/hβS-scr; #P < 0.05, ###P < 0.001 vs. hβA/hβA-siRNA. n = 8/group. (D) When tested on day 4, lidocaine produced CPP in TOW mice treated with RVG-9R/scrambled (scr) RNA duplex, not RVG-9R/PKCδ-siRNA. ***P < 0.001. n = 6/group. (E) Mechanical allodynia and (F) thermal hyperalgesia developed in PKCδ-WT mice, not PKCδ-KO mice, after hematopoietic stem cell transplant from donor TOW mice. ***P < 0.001 vs. pre. n = 6/group. (G) Lidocaine produced CPP paradigm in PKCδ-WT mice, not PKCδ-KO mice, 4 weeks after transplant. ***P < 0.001. n = 6/group. Data were analyzed by ANOVA followed by Dunnett’s t test. Two-way ANOVA (pairing vs. treatment) and post hoc Bonferroni’s test were used to analyze CPP data