Abstract
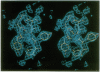
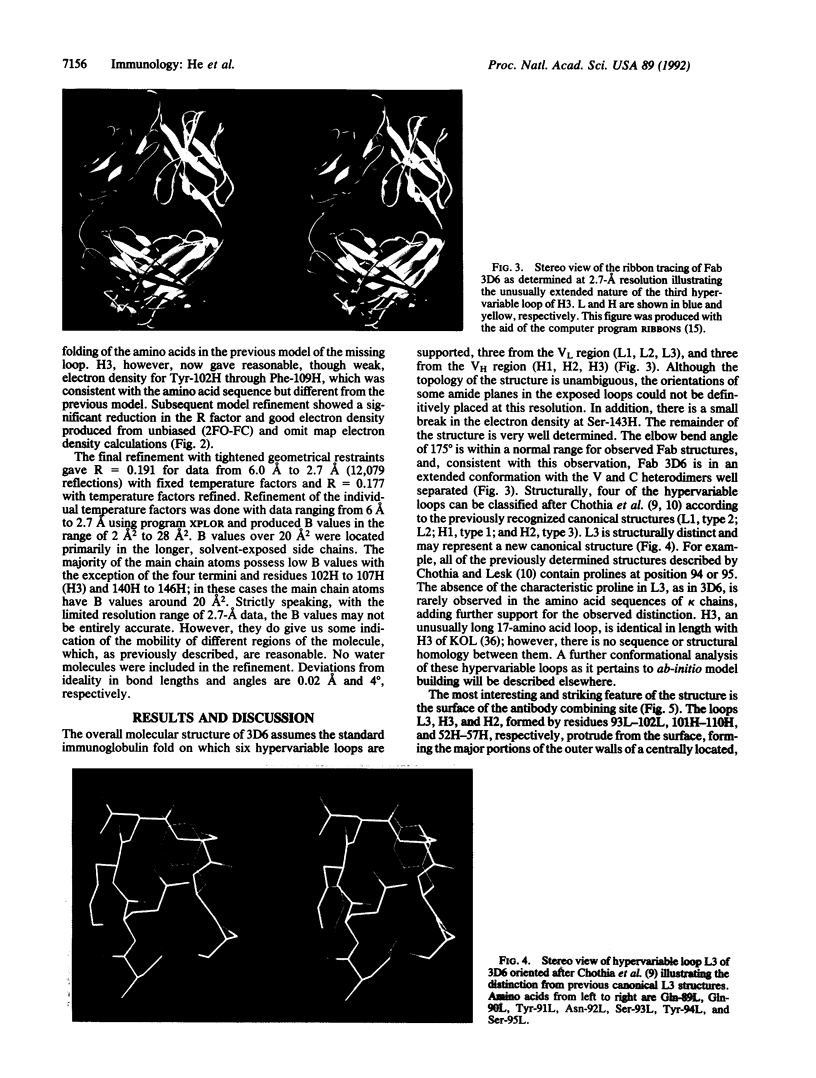
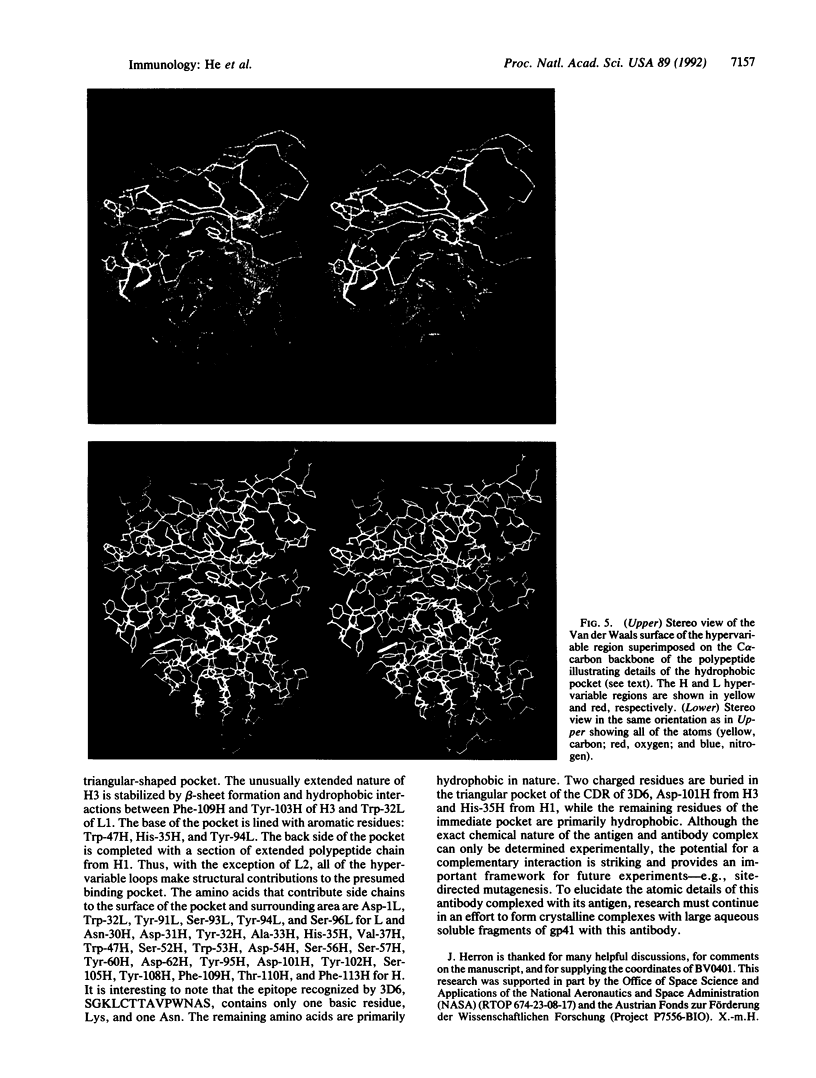
The three-dimensional structure of a human monoclonal antibody (Fab), which binds specifically to a major epitope of the transmembrane protein gp41 of the human immunodeficiency virus type 1, has been determined by crystallographic methods to a resolution of 2.7 A. It has been previously determined that this antibody recognizes the epitope SGKLICTTAVPWNAS, belongs to the subclass IgG1 (kappa), and exhibits antibody-dependent cellular cytotoxicity. The quaternary structure of the Fab is in an extended conformation with an elbow bend angle between the constant and variable domains of 175 degrees. Structurally, four of the hypervariable loops can be classified according to previously recognized canonical structures. The third hypervariable loops of the heavy (H3) and light chain (L3) are structurally distinct. Hypervariable loop H3, residues 102H-109H, is unusually extended from the surface. The complementarity-determining region forms a hydrophobic binding pocket that is created primarily from hypervariable loops L3, H3, and H2.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amit A. G., Mariuzza R. A., Phillips S. E., Poljak R. J. Three-dimensional structure of an antigen-antibody complex at 2.8 A resolution. Science. 1986 Aug 15;233(4765):747–753. doi: 10.1126/science.2426778. [DOI] [PubMed] [Google Scholar]
- Bentley G. A., Boulot G., Riottot M. M., Poljak R. J. Three-dimensional structure of an idiotope-anti-idiotope complex. Nature. 1990 Nov 15;348(6298):254–257. doi: 10.1038/348254a0. [DOI] [PubMed] [Google Scholar]
- Bird R. E., Hardman K. D., Jacobson J. W., Johnson S., Kaufman B. M., Lee S. M., Lee T., Pope S. H., Riordan G. S., Whitlow M. Single-chain antigen-binding proteins. Science. 1988 Oct 21;242(4877):423–426. doi: 10.1126/science.3140379. [DOI] [PubMed] [Google Scholar]
- Bruccoleri R. E., Karplus M. Prediction of the folding of short polypeptide segments by uniform conformational sampling. Biopolymers. 1987 Jan;26(1):137–168. doi: 10.1002/bip.360260114. [DOI] [PubMed] [Google Scholar]
- Brünger A. T., Kuriyan J., Karplus M. Crystallographic R factor refinement by molecular dynamics. Science. 1987 Jan 23;235(4787):458–460. doi: 10.1126/science.235.4787.458. [DOI] [PubMed] [Google Scholar]
- Casale E., Wenisch E., He X. M., Righetti P. G., Snyder R. S., Jungbauer A., Tauer C., Ruker F., Carter D. C. Crystallization of the Fab from a human monoclonal antibody against gp 41 of human immunodeficiency virus type I. J Mol Biol. 1990 Dec 5;216(3):511–512. doi: 10.1016/0022-2836(90)90377-X. [DOI] [PubMed] [Google Scholar]
- Chothia C., Lesk A. M. Canonical structures for the hypervariable regions of immunoglobulins. J Mol Biol. 1987 Aug 20;196(4):901–917. doi: 10.1016/0022-2836(87)90412-8. [DOI] [PubMed] [Google Scholar]
- Chothia C., Lesk A. M., Tramontano A., Levitt M., Smith-Gill S. J., Air G., Sheriff S., Padlan E. A., Davies D., Tulip W. R. Conformations of immunoglobulin hypervariable regions. Nature. 1989 Dec 21;342(6252):877–883. doi: 10.1038/342877a0. [DOI] [PubMed] [Google Scholar]
- Colman P. M., Laver W. G., Varghese J. N., Baker A. T., Tulloch P. A., Air G. M., Webster R. G. Three-dimensional structure of a complex of antibody with influenza virus neuraminidase. 1987 Mar 26-Apr 1Nature. 326(6111):358–363. doi: 10.1038/326358a0. [DOI] [PubMed] [Google Scholar]
- Colman P. M., Tulip W. R., Varghese J. N., Tulloch P. A., Baker A. T., Laver W. G., Air G. M., Webster R. G. Three-dimensional structures of influenza virus neuraminidase-antibody complexes. Philos Trans R Soc Lond B Biol Sci. 1989 Jun 12;323(1217):511–518. doi: 10.1098/rstb.1989.0028. [DOI] [PubMed] [Google Scholar]
- Davies D. R., Sheriff S., Padlan E. A. Antibody-antigen complexes. J Biol Chem. 1988 Aug 5;263(22):10541–10544. [PubMed] [Google Scholar]
- Döpel S. H., Porstmann T., Henklein P., von Baehr R. Fine mapping of an immunodominant region of the transmembrane protein of the human immunodeficiency virus (HIV-1). J Virol Methods. 1989 Aug;25(2):167–177. doi: 10.1016/0166-0934(89)90030-x. [DOI] [PubMed] [Google Scholar]
- Felgenhauer M., Kohl J., Rüker F. Nucleotide sequences of the cDNAs encoding the V-regions of H- and L-chains of a human monoclonal antibody specific to HIV-1-gp41. Nucleic Acids Res. 1990 Aug 25;18(16):4927–4927. doi: 10.1093/nar/18.16.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glockshuber R., Steipe B., Huber R., Plückthun A. Crystallization and preliminary X-ray studies of the VL domain of the antibody McPC603 produced in Escherichia coli. J Mol Biol. 1990 Jun 20;213(4):613–615. doi: 10.1016/S0022-2836(05)80247-5. [DOI] [PubMed] [Google Scholar]
- Grunow R., Jahn S., Porstmann T., Kiessig S. S., Steinkellner H., Steindl F., Mattanovich D., Gürtler L., Deinhardt F., Katinger H. The high efficiency, human B cell immortalizing heteromyeloma CB-F7. Production of human monoclonal antibodies to human immunodeficiency virus. J Immunol Methods. 1988 Feb 10;106(2):257–265. doi: 10.1016/0022-1759(88)90206-2. [DOI] [PubMed] [Google Scholar]
- Hendrickson W. A. Stereochemically restrained refinement of macromolecular structures. Methods Enzymol. 1985;115:252–270. doi: 10.1016/0076-6879(85)15021-4. [DOI] [PubMed] [Google Scholar]
- Herron J. N., He X. M., Ballard D. W., Blier P. R., Pace P. E., Bothwell A. L., Voss E. W., Jr, Edmundson A. B. An autoantibody to single-stranded DNA: comparison of the three-dimensional structures of the unliganded Fab and a deoxynucleotide-Fab complex. Proteins. 1991;11(3):159–175. doi: 10.1002/prot.340110302. [DOI] [PubMed] [Google Scholar]
- Huston J. S., Levinson D., Mudgett-Hunter M., Tai M. S., Novotný J., Margolies M. N., Ridge R. J., Bruccoleri R. E., Haber E., Crea R. Protein engineering of antibody binding sites: recovery of specific activity in an anti-digoxin single-chain Fv analogue produced in Escherichia coli. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5879–5883. doi: 10.1073/pnas.85.16.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungbauer A., Tauer C., Wenisch E., Steindl F., Purtscher M., Reiter M., Unterluggauer F., Buchacher A., Uhl K., Katinger H. Pilot scale production of a human monoclonal antibody against human immunodeficiency virus HIV-1. J Biochem Biophys Methods. 1989 Aug-Sep;19(2-3):223–240. doi: 10.1016/0165-022x(89)90029-8. [DOI] [PubMed] [Google Scholar]
- Marquart M., Deisenhofer J., Huber R., Palm W. Crystallographic refinement and atomic models of the intact immunoglobulin molecule Kol and its antigen-binding fragment at 3.0 A and 1.0 A resolution. J Mol Biol. 1980 Aug 25;141(4):369–391. doi: 10.1016/0022-2836(80)90252-1. [DOI] [PubMed] [Google Scholar]
- Martin A. C., Cheetham J. C., Rees A. R. Modeling antibody hypervariable loops: a combined algorithm. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9268–9272. doi: 10.1073/pnas.86.23.9268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A. C., Cheetham J. C., Rees A. R. Molecular modeling of antibody combining sites. Methods Enzymol. 1991;203:121–153. doi: 10.1016/0076-6879(91)03008-5. [DOI] [PubMed] [Google Scholar]
- Padlan E. A., Silverton E. W., Sheriff S., Cohen G. H., Smith-Gill S. J., Davies D. R. Structure of an antibody-antigen complex: crystal structure of the HyHEL-10 Fab-lysozyme complex. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5938–5942. doi: 10.1073/pnas.86.15.5938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts S., Cheetham J. C., Rees A. R. Generation of an antibody with enhanced affinity and specificity for its antigen by protein engineering. Nature. 1987 Aug 20;328(6132):731–734. doi: 10.1038/328731a0. [DOI] [PubMed] [Google Scholar]
- Saul F. A., Amzel L. M., Poljak R. J. Preliminary refinement and structural analysis of the Fab fragment from human immunoglobulin new at 2.0 A resolution. J Biol Chem. 1978 Jan 25;253(2):585–597. [PubMed] [Google Scholar]
- Sheriff S., Silverton E. W., Padlan E. A., Cohen G. H., Smith-Gill S. J., Finzel B. C., Davies D. R. Three-dimensional structure of an antibody-antigen complex. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8075–8079. doi: 10.1073/pnas.84.22.8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skerra A., Glockshuber R., Plückthun A. Structural features of the McPC603 Fab fragment not defined in the X-ray structure. FEBS Lett. 1990 Oct 1;271(1-2):203–206. doi: 10.1016/0014-5793(90)80406-9. [DOI] [PubMed] [Google Scholar]
- Skerra A., Plückthun A. Assembly of a functional immunoglobulin Fv fragment in Escherichia coli. Science. 1988 May 20;240(4855):1038–1041. doi: 10.1126/science.3285470. [DOI] [PubMed] [Google Scholar]
- Stanfield R. L., Fieser T. M., Lerner R. A., Wilson I. A. Crystal structures of an antibody to a peptide and its complex with peptide antigen at 2.8 A. Science. 1990 May 11;248(4956):712–719. doi: 10.1126/science.2333521. [DOI] [PubMed] [Google Scholar]
- Waldmann T. A. Monoclonal antibodies in diagnosis and therapy. Science. 1991 Jun 21;252(5013):1657–1662. doi: 10.1126/science.2047874. [DOI] [PubMed] [Google Scholar]