Abstract
Aims:
To review the burden of allergic and infectious diseases and the evidence for a link to microbial exposure, the human microbiome and immune system, and to assess whether we could develop lifestyles which reconnect us with exposures which could reduce the risk of allergic disease while also protecting against infectious disease.
Methods:
Using methodology based on the Delphi technique, six experts in infectious and allergic disease were surveyed to allow for elicitation of group judgement and consensus view on issues pertinent to the aim.
Results:
Key themes emerged where evidence shows that interaction with microbes that inhabit the natural environment and human microbiome plays an essential role in immune regulation. Changes in lifestyle and environmental exposure, rapid urbanisation, altered diet and antibiotic use have had profound effects on the human microbiome, leading to failure of immunotolerance and increased risk of allergic disease. Although evidence supports the concept of immune regulation driven by microbe–host interactions, the term ‘hygiene hypothesis’ is a misleading misnomer. There is no good evidence that hygiene, as the public understands, is responsible for the clinically relevant changes to microbial exposures.
Conclusion:
Evidence suggests a combination of strategies, including natural childbirth, breast feeding, increased social exposure through sport, other outdoor activities, less time spent indoors, diet and appropriate antibiotic use, may help restore the microbiome and perhaps reduce risks of allergic disease. Preventive efforts must focus on early life. The term ‘hygiene hypothesis’ must be abandoned. Promotion of a risk assessment approach (targeted hygiene) provides a framework for maximising protection against pathogen exposure while allowing spread of essential microbes between family members. To build on these findings, we must change public, public health and professional perceptions about the microbiome and about hygiene. We need to restore public understanding of hygiene as a means to prevent infectious disease.
Keywords: allergy, infectious disease, hygiene, cleaning, antibiotics, diet
Introduction
Allergic diseases including asthma, hay fever, eczema and food allergies have dramatically increased over the last century, initially in high-income communities but now elsewhere. At the same time, threats of infectious disease pandemics, antibiotic resistance and numbers of immune-compromised people living in the community have increased. Taken together, these diseases are a significant burden on health and prosperity.
The idea that there might be a link between the rise in allergic disease and reduced microbial exposure as a result of measures introduced to protect against infection was first proposed in 1989.1,2 This so-called hygiene hypothesis, as outlined by Dr David Strachan, proposed that a lower incidence of infection in early childhood could be an explanation for the 20th century rise in atopic diseases. Although a simple idea in itself, it raised the thought that rising allergies may be an inevitable price to be paid for freedom from the burden of killer infectious diseases. Although evidence still supports the concept that immune regulation is driven by microbe–host interactions, the term ‘hygiene hypothesis’ is now being seen by many as a misleading misnomer for a concept with far-reaching consequences for public health and an issue which needs to be addressed.3,4
Humans are ecosystems, where the microbes that live on and within us (the human microbiome) constitute an organ at least as essential to health as our liver or kidneys.5 The immune system is a learning device, and at birth it resembles a computer with hardware and software but few data. Additional data must be supplied during the first years of life, through contact with microorganisms from other humans and the natural environment. If these inputs are inadequate or inappropriate, the regulatory mechanisms of the immune system can fail. As a result, the system attacks not only harmful organisms which cause infections but also innocuous targets such as pollen, house dust and food allergens resulting in allergic diseases.
Despite this new understanding, the hygiene hypothesis concept – that we have become too clean – still persists in the minds of the public. As a result, the public has lost confidence in hygiene. This is happening at a time when infectious disease issues mean that hygiene is becoming more, rather than less, important.
The aim of this study is to review the burden of allergic and infectious diseases and the evidence for a link to microbial exposure, the human microbiome and immune system. Also, it is to assess whether and to what extent we could develop lifestyles which reconnect us with exposures and thereby reduce the risks of allergic disease while also protecting against infectious disease.
Methods
Using methodology based on the Delphi technique,6–9 six experts in infectious and allergic disease were surveyed to allow for elicitation of group judgement in order to arrive at a consensus view on issues pertinent to the aim of the study.
Key themes emerged: first, the extent of the health burden of allergic and hygiene-related diseases; second, the most recent evidence regarding the nature of the link between reduced microbial exposure and its impact on the human microbiome and the immune regulatory system; third, the question of relationship between lifestyles and protection against infectious diseases. The Delphi technique is a qualitative research method that relies on the judgement of individuals presumed to be knowledgeable and expert at what they do. When a sufficient degree of consensus is achieved, the Delphi process is curtailed and the resulting judgement is published. Six experts in infectious diseases and allergies were invited to participate, and the issues to be addressed were agreed via online communication. The authors participated in a conference in which each presented evidence related to their area of expertise. Following this, authors submitted a written contribution. These were analysed and key themes were integrated into a paper which was made available online to all authors for review. This included further questions soliciting the author’s views. After further rounds of questions and revision, a consensus position was obtained.
Results
Why hygiene is important in the 21st century
In the 1950s and 1960s, there was optimism that, with vaccination and antibiotics freely available, conquest of most infections would follow. During the last four decades, this opinion has been reversed. Infectious disease continues to exert a heavy burden on health and prosperity. The various infectious disease issues are most often considered in isolation, but when viewed together, they represent a powerful argument for renewed emphasis on hygiene, which alongside vaccination strategies remain key to containing infectious disease.10
During the 1980s, there was a rapid increase in reported cases of food poisoning in the United Kingdom, particularly related to Salmonella and Campylobacter.11 Although reported cases have somewhat declined, food, waterborne, and non-food-related infectious intestinal diseases (IIDs) remain at unacceptable levels. The latest study of IID (food and non-foodborne IID) reported that the true incidence in the community is 43% higher than in the mid-1990s: this study estimated 17 million cases a year in the United Kingdom.12 The estimated cost of food-related IID is £1.5 billion a year, including resource and welfare losses.12 Norovirus, mainly spread from person-to-person, is the most significant cause of intestinal infections in the developed world, including 3 million cases per year in the United Kingdom.12
Evidence shows that respiratory hygiene involving hands and surfaces can limit spread of respiratory infections, particularly colds, and also influenza.13–15 Since respiratory and intestinal viral infections are not treatable by antibiotics, prevention through hygiene is key.
In developed countries, about 7% of inpatients acquire an infection in hospital.16 Recent figures show a decline in health-care-associated infection (HCAI), in the United Kingdom, particularly of Clostridium difficile and MRSA (methicillin-resistant Staphylococcus aureus),17,18 while other causes of HCAI have emerged, including new epidemic strains of Escherichia coli, Pseudomonas spp. and viruses.
Governments, looking at prevention as a means to reduce health spending, have introduced shorter hospital stays and increased homecare. This requires new policies to prevent HCAIs in community settings19 where there is no evidence of a decline. Until recently, most episodes of C. difficile infection were believed to result from acquisition in health-care settings. There is now increasing evidence of multiple other potential sources, including asymptomatic patients, and sources in the wider environment, such as water, farm animals or pets, and food.20 The contribution of cases acquired from these sources to the overall burden of disease is unclear, particularly with concerns about increased community-associated C. difficile infection.21
Societal changes mean that people with greater susceptibility to infectious disease make up an increasing proportion of the population, up to 20% or more.10 The largest proportion comprises the elderly who have reduced immunity, often exacerbated by other illnesses. It also includes the very young and family members with invasive devices such as catheters and people whose immuno-competence is impaired as a result of chronic and degenerative illness (including HIV/AIDS) or drug therapies such as cancer chemotherapy.
Emerging pathogens and new strains are a significant concern. It is remarkable that norovirus, Campylobacter and Legionella were largely unknown as human pathogens before the 1970s, with others such as E. coli O157 and O104 emerging in subsequent decades. It is now thought likely that we shall identify many more, the latest being Zika virus.22 Agencies worldwide recognise that for threats such as new influenza strains, SARS (severe acute respiratory syndrome) and Ebola, hygiene is a first line of defence during the early critical period before mass measures such as vaccination become available.23 The low infectious dose observed for several of the emerging pathogens, such as E. coli O157:H7 and norovirus, is an additional concern that emphasises the role that hygiene can play in prevention.24,25
Antibiotic resistance is a global priority.26 Hygiene addresses this problem by reducing the need for antibiotic prescribing and reducing ‘silent’ spread of antibiotic resistant strains in the community and hospitals.27 As persistent nasal or bowel carriage of these strains spreads in the healthy population, this increases the risk of infection with resistant strains in both hospitals and the community.27
Infections can act as co-factors in diseases, such as cancer and chronic degenerative diseases. Syndromes such as Guillain–Barré28 and triggering of allergy by viral infections29 add to the burden of hygiene-related infection.
The rise of allergies in the 20th century
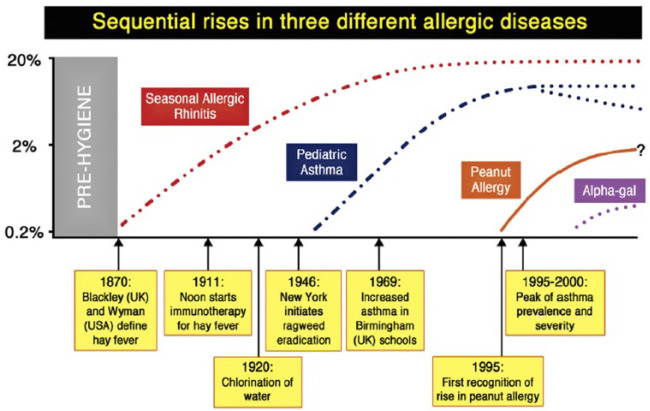
While infectious disease and hygiene have been key public health issues for centuries,30 allergic diseases have only relatively recently been regarded as a significant health burden. The marked increase in prevalence of allergic diseases, such as eczema,31 allergic rhinitis32 and food allergy,32 has been a prominent trend over the past century in all regions of the world, but most characterised in Western countries.33 While this is frequently presented as an ‘epidemic’, epidemiological data indicate the situation is more complex. As highlighted by Platts-Mills34 (Figure 1), the ‘spikes’ in prevalence of allergic rhinitis, asthma and food allergy have occurred at different times in the past 120 years, and thus different atopic diseases may have different contributing factors. Indeed, there are emerging data that in some areas (mostly in ‘Western’ countries) these increases may have plateaued and even begun to subside.34 A further issue is that at least for food allergy, prevalence may have been overestimated, depending on the methodology used. Venter et al.35 assessed the rate of challenge-positive food allergy in three birth cohorts on the Isle of Wight (UK) between 1989 and 2002. A major finding of this study, confirmed in other reports, is that rates of parent-reported allergy were significantly higher (33%) than those confirmed by placebo-controlled food challenge (6%) (the accepted gold standard for diagnosis). For peanut allergy, the same study reported a rate of 1 in 200 children aged 3–4 years in 1989, increasing to 1 in 70 in the mid-1990s, but plateauing thereafter. A 2016 UK intervention study, in children breast-fed to at least 6 months of age, reported a rate of 1 in 40.36 Of note, the development of an inappropriate immune response to foods (‘sensitisation’), which occurs before onset of clinical disease, is an early event often occurring in the first few months of life.36
Figure 1.
Trends in allergic disease
Reprinted from Platts-Mills,34 Copyright (2015), with permission from American Academy of Allergy, Asthma & Immunology and Elsevier
Perhaps the relatively late appearance of food allergy over the past few decades is a consequence of a progression from allergic airways disease (hay fever, asthma) in parents to a more severe clinical phenotype (food allergy) in their offspring.37 However, a compelling alternative is the interaction between genetic predisposition and environmental influences, particularly for food allergy, where immune sensitisation to foods may originate with exposure to food allergens in the environment through the skin, a situation exacerbated by eczema and reduced skin barrier function.36 At the same time, there have been changes in how foods are consumed (e.g. roasted peanut, as consumed in Europe and North America, is more allergenic than raw or other forms of processed peanut).
From Hygiene Hypothesis to Old Friends Mechanism
Building on the significant amount of research published since 1989, a number of refinements to the original hygiene hypothesis now seem to offer more plausible explanations. The Old Friends (OF) Mechanism was proposed by Rook in 2003 and argues that the vital microbial exposures are not colds, measles and other childhood infections (the crowd infections), but rather microbes already present during primate evolution and in hunter-gatherer times when the human immune system was evolving.38–40 OF microbes include environmental species which inhabit indoor and outdoor environments, and the largely non-harmful commensal microbes acquired from the skin, gut and respiratory tract of other humans. In evolving humans, before the advent of modern medicine, the OF also included organisms such as helminths, Helicobacter pylori, and hepatitis A virus that could persist for life in hunter-gatherer groups and that needed to be tolerated. They all therefore activated immunoregulatory mechanisms,38 but few experts believe that they need to be replaced or even that there is any feasible way of doing so.
Whereas the hygiene hypothesis implicated childhood virus infections as the vital exposures, from an evolutionary point of view this was never likely. Crowd infections were not part of human evolutionary experience because they either kill or induce solid immunity, so could not persist in small hunter-gatherer groups.41 Epidemiological studies carried out in Finland, Denmark and the United Kingdom now confirm that childhood infections do not protect against allergic disorders.42–44
Studies show how OF exposures are vital because they interact with the regulatory systems that keep the immune system in balance and prevent overreaction, which is an underlying cause of allergies. Diversity of microbial exposure is key. First, a large experience of harmless bacteria and archaea during infancy, when immunoregulatory systems are being established, increases the repertoire of organisms that can be tolerated. Second, since all life-forms are ultimately constructed from similar building blocks, exposed individuals acquire some memory lymphocytes that recognise novel pathogens or even novel viruses.45
What are the likely causes of reduced or altered microbial exposures
In order to look for strategies which might restore the necessary microbial exposures, it is first necessary to understand the underlying causes of the loss of exposure. Since allergic diseases are largely conditions of the last 100 years, an obvious assumption is that the sanitary revolution is a root cause. The latter part of the 19th century saw radical improvements in sanitation, cleaner food and water, clean-up of cities, and rapid decline in infectious diseases.46 However, it is likely that these changes also inadvertently reduced exposure to OF microbes which occupy the same habitats. Since the major changes in water, sanitation and hygiene had occurred by 1920, it is difficult to ascribe the massive changes in the asthma prevalence from 1960 onwards to these changes.34
It is now clear that the most important times for OF exposure are early in development, during pregnancy, delivery, and the first few days or months of infancy.47,48 A 2008 review of epidemiological studies show that Caesarean section is linked to increased risk of allergy.49 C-sections have become increasingly common since 1950 and now account for 25% of UK births.50 Furthermore, transfer of microbiota occurs via the mother’s milk, which is not sterile.51 Breast versus bottle feeding has a large influence on gut microbiome,52,53 but further studies are needed to confirm any association with allergic disease. In high-income settings, there is likely to be a trans-generational effect where each generation receives a more impoverished microbiota, and essential microbiota are lost from the community.54
Continuing early-life exposure from the mother and siblings is also important.55,56 Studies show that children from large families are at lower risk of developing allergies.52,57 Exposure to pets protects against allergies,58,59 although domestic animals in the home have increased rather than decreased.60 People seem to share their microbiota via dogs,61 which greatly increase the microbial biodiversity of the home.62,63
There is good evidence that contact with microbial diversity from the natural environment is crucial. Numerous studies now show that exposure to farm environments during the first 2–3 years of life protects against allergic disorders64–66 and correlates with microbial biodiversity in the air67 and the home.68 Animal models show candidate organisms from these environments protect against allergic disorders.69 Studies in Finland show that living close to green space and agriculture rather than close to a town increases biodiversity of the skin microbiota and correlates with reduced allergic sensitisation.70 Urbanisation has accelerated loss of exposure to the natural environment. In the United Kingdom, 82% of the population now live in urban areas,71 with up to 90% of our time spent indoors.72
Although research has tended to focus on the gut microbiome, it seems likely that the microbiome of skin and airways is also involved.73–75 Much of the exposure obtained from outdoor environments is likely to be via the airways. The air contains bacteria, archaea, viruses, fungi, spores, pollen, plant biomass and dust. Depending on the environment and on degree of exertion, the number of bacteria/archaea breathed in could vary between about 106 and 1010 in 24 h. A proportion of these will be retained in the airways, and recent work reveals that exposure to bacterial components causes increased expression of a protein that inhibits inflammation.73,74 Gut exposure is also mediated via the airways where ciliary action brings about transfer to the gut. The likelihood that skin microbiota are OF microbes is indicated by studies showing that Acinetobacter species in skin protect against allergy.75
Factors that maintain the gut microbiota
Once the microbiome has been acquired and evolved during childhood,48 the critical question becomes what factors maintain optimum composition and biodiversity, because loss of biodiversity is strongly associated with disease states, inflammation and decline.76–78
Increasingly, the answer appears to be that the optimal composition of the microbiota is maintained by diet,79 which needs to be diverse, and contain fibre (polysaccharides digested by the microbiota rather than the human host),80 and polyphenols found in plant products.81–83 A diet deficient in fibre can lead to progressive extinctions of important groups of organisms,54 which are cumulative and increasingly difficult to reverse in subsequent generations.42 Polyphenols and also fish oils also appear to modulate the composition of the microbiota.84,85
Citizens of high-income countries have less diverse microbiota than do hunter-gatherers.77–79 Other studies show that the elderly living in the community with healthy diets78 have higher gut microbiota diversity than those in long-stay residential care who have a less diverse diet. Studies in Sweden and Denmark show that reduced gut microbiota diversity in infants is associated with increased risk of allergic disease in childhood.86–88
Introduction of antibiotics in the 1950s and subsequent prescribing trends, show a compelling temporal fit with rising allergies since the 1970s. A 2014 review of evidence from over 50 epidemiological studies shows a reasonably consistent relationship between excessive antibiotic use, particularly in early childhood, and increased risk of allergic disease.89 Evidence showing that exposure to antibiotics during pregnancy increases the risk of allergic disorders in infants90,91 has been further confirmed in recent studies.92,93 Antibiotics, particularly macrolides, have lasting effects on the microbiota of young children and increase risks of asthma.92 This mirrors effects documented in animal models, where early disruption of gut microbiota causes long-term damage to metabolic regulation.94
Disruptions of maternal microbiota diversity by antibiotics or inadequate diet are found to be transmitted to future generations.54
Domestic and personal hygiene
Of all the trends that might explain declining OF exposure, one of the weakest is the popular notion of ‘being too clean in our own homes’. If this factor contributes, its role is likely to be small relative to other factors. An explosion of data, obtained using high-throughput RNA sequencing of samples from US homes, suggests that modern homes are ‘teeming with microbes’. It also suggests that the bacterial communities found in the home relate to the people and domestic animals living there and the food they eat, together with input from the local outdoor environment.63,95
Microbiological studies in westernised homes indicate that routine daily or weekly cleaning habits (even involving use of antibacterial cleaners) have no sustained effect on levels of microbes in our homes.96–98 The idea that we could create ‘sterile’ homes through excessive cleanliness is implausible; as fast as microbes are removed, they are replaced, via dust and air from the outdoor environment, and commensal microbes shed from the human body and our pets, and contaminated foods brought into the homes. Strachan’s1 1989 proposition that ‘higher standards of personal cleanliness’ could also contribute to reduced exposure to essential microbes may be compatible with increased bathing/showering/shampooing since around 1950s,46 but although bathing and so on removes large numbers of microbes from the skin, these are rapidly replaced.
Although data from westernised homes suggest that more diverse communities can be found on less-cleaned surfaces (TV screen, door trims, floors) than regularly cleaned surfaces (cutting board, kitchen surface, toilet seat),63,95 to date, there is no confirmed evidence of a link between personal or home cleanliness and increased risk of allergic disease. In a German birth cohort study of 399 families, personal cleanliness (e.g. handwashing and showering) was associated with lower levels of endotoxin and muramic acid (bacterial markers) in bedding and floor dust. In comparison, household cleanliness (e.g. cleaning floors and bathrooms, dusting, and changing towels) was associated with less dust but not with lower microbial marker levels. Endotoxin in infancy was associated with less allergic sensitisation and less asthma when these children reached school age, whereas muramic acid exposure at school age, but not infancy, was associated with less school-age asthma and eczema.99 It might seem surprising that neither personal nor home cleanliness activities were directly associated with allergy outcomes, but Liu100 suggests that this may reflect the importance of early-life timing of microbial exposures and not cleanliness behaviours, with the influence of endotoxin exposure being in infancy. A 2002 data analysis of UK children born in 1991/1992 found association between parent-reported frequency of hand and face washing, showering and bathing at 15 months and wheezing and atopic eczema at 30–42 months, but this association was not reported in other studies.101,102
The key point may be that the microbial content of modern urban homes has altered relative to earlier generations, not because of home and personal cleanliness but because, prior to the 1800s, people lived in predominantly rural surroundings. Also, although human gut and skin microbiota are constantly shed from family members, it is likely that exposure has altered reflecting the reduced diversity of the human microbiota due to factors described above. This means we now interact with an altogether different and less diverse mix of microbes.
Other factors also argue against the role of hygiene. Hygiene is irrelevant to microbiome disruption through altered diet and antibiotics. Also, if contact with the natural environment and microbial components of house dust occurs mostly via the airways, hygiene and cleanliness is unlikely to be responsible for reduced inputs from this key source.
Communicating Microbiome Science to Society – Prelude to Reversing Immunoallergic Disorders
Although evidence suggests that strategies such as promoting natural childbirth and breast feeding, increased social exposure through sport, other outdoor activities, less time spent indoors, diet and appropriate antibiotic use could help restore the microbiome and perhaps reduce risks of allergic disease, clinical and other evaluations are required to establish whether and to what extent this might occur and when intervention is most beneficial.
There is a window of time when the developing microbiome is critical for the education of the maturing immune system. Disruption or delay in acquisition of the microbiome in the first few years of life may predispose to later immune dysfunction. It follows that preventive efforts against immuno-allergic disorders must be focussed on early life events. Attempts to correct abnormal host–microbe interactions, once immunological events which lead to allergic disease are established, may be too late. These issues are further discussed by Shanahan and colleagues.103–105 Gaps in understanding host–microbe interactions will be addressed as research continues, and one can anticipate a time, when optimal conditions for colonisation of the newborn are understood and can be controlled by strategies ensuring neonates begin life with a robust and diverse microbiota. In the interim, there is much that can be achieved by education and behaviour change, based on current information.
Several factors seem to conspire to limit effective communication of microbiome science to society (Table 1). Some elements within the popular media do disservice to their readership. Examples include mis-representation of the role of hygiene and cleanliness, failure to clarify that probiotics are not all the same, and failure to probe unsubstantiated health claims or address seemingly complex concepts in detail. Fault also lies elsewhere (Table 1). In contrast to policy makers and public health officials, clinicians deal with individual patients, not populations. Unless concerns about antibiotic usage are brought to an individual level, with emphasis on the consumer rather than the prescriber, reform initiatives will have limited impact. Patients are less likely to demand antibiotics if provided with information on the impact of such agents on the microbiota and the risk of immune disorders in later life.106
Table 1.
Science and society – communication barriers
| The stakeholders | Challenge |
|---|---|
| Media | Preoccupation with sensationalism rather than
truth Over-simplification and mis-portrayal of concepts such as hygiene, probiotics, and microbiota Assumption that the readership cannot understand complex concepts |
| Medical/clinical professionals | Lagging behind the science Medical curricula focus on threat of infection rather than benefits of indigenous microbiota Inadequately equipped to address patients’ questions on information acquired from the media Inadequate response by the medical establishment to inaccurate and misleading material presented in the media |
| Scientists | Excessive use of hyperbole Poor language Failure to standardise terminology and methodology |
| Policy makers and Public health officials | Ineffective and mixed messages to the public Excessive focus on antibiotic resistance rather than risk of collateral damage to microbiota in promoting judicious use of antibiotics Poor communication of influence of diet on microbiota |
| Lay public | Poor conceptualisation of risk versus
benefit Inadequately served by media for appraisal of medical and scientific claims |
Promotion of breast feeding is lacking in precise rationale for modern women. Breast-feeding mothers need to know they are promoting a lifelong healthy microbiota for their offspring. Since the neonate acquires its microbiome primarily from its mother, greater attention needs to be paid to the mother’s diet, faecal and vaginal microbiome. Increasing awareness of the importance of the microbiome and the factors which sustain or disrupt it should be part of antenatal education.
Microbiome science already provides a glimpse of how the microbiota may be preserved or restored, including development of smart antibiotics,107 non-antibiotic anti-microbials, microbial transplants, microbial consortia or single strains, and use of personalised biomarkers of disease risk prediction.108,109 Restoration of the microbiome by vaginal microbiota transplants in C-section infants has been demonstrated,110 albeit of unproven long-term benefit and controversial.111 In addition, the molecular basis by which bifidobacteria engage with the host immune system is emerging;112,113 this is important because such organisms are a predominant component of the microbiota in neonates.
Because of the multiplicity of factors involved, strategies to preserve or manipulate the microbiota will probably require a personalised approach tailored to individual genetics and lifestyle factors.109
Developing and Promoting A Targeted Approach to Hygiene in Home and Everyday Life
Over the last 20 years or so, for reasons outlined above, there has not only been a revival of concern about infection and the role of hygiene10,114 but also a realisation that the ‘scrupulous cleanliness’ approach advocated by Florence Nightingale115 is no longer appropriate. If, as this review suggests, allergic diseases are not the price we have to pay for protection against infection, this is good news for hygiene. However, if we are to maximise protection against infection while at the same time sustaining exposure to essential microbes, we need a revised approach to hygiene based on current scientific evidence.
The International Scientific Forum on Home Hygiene (IFH) (http://www.ifh-homehygiene.org) was established in 1997 with the aim of developing and promoting a more effective approach to hygiene, based on scientific principles and the growing database of evidence about pathogen transmission.116 To achieve this, IFH adopted the principle of targeted hygiene.117 Targeted Hygiene is based on a four-step risk assessment requiring identification of the sources and reservoirs of pathogens, the routes of transmission, the critical control points, and appropriate hygiene interventions.
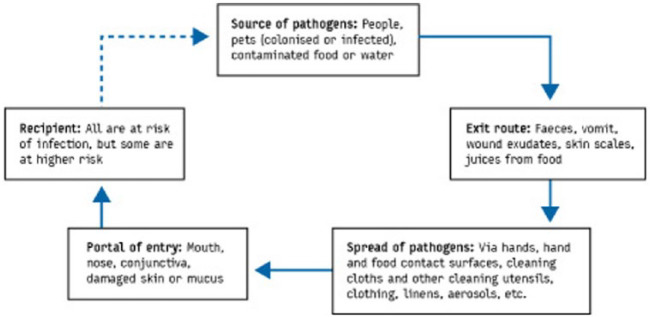
Targeted hygiene is based on the chain of infection transmission (Figure 2) which shows that pathogenic organisms are continually shed into the environment from sources such as human occupants, pets and raw foods.118
Figure 2.
The chain of infection transmission in the home
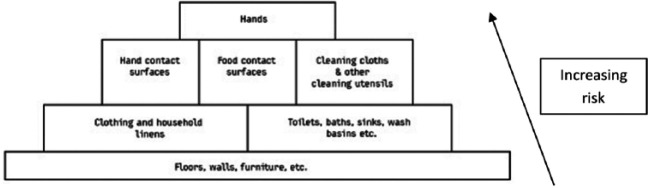
To get from an infected source to another individual, pathogens use well defined routes. Sampling studies record the presence of non-pathogenic bacteria and bacteria and viruses of medical interest on environmental surfaces in home and community settings, and laboratory and field studies have evaluated the rates of transfer of viral and bacterial pathogens via hands and common touch surfaces.116 These demonstrate that the critical control points for transmission of infection are the hands, hand contact surfaces, food contact surfaces, and cleaning utensils and that these present the highest risk of transmission (Figure 3).
Figure 3.
Ranking of sites and surfaces based on risk of transmission of infection
Equally important considerations are the interventions used to eliminate pathogens from critical control points before they spread further. This is important since inadequate procedures can increase transmission.119–123 Hygienic (as opposed to visible) cleaning of hands, surfaces, fabrics and so on can be achieved by the following:
Physical removal of pathogens from inanimate or skin surfaces using soap or detergent-based cleaning. To be effective as a hygiene measure, this should be accompanied with thorough rinsing under running water, such that pathogens are not further disseminated.
Using an antimicrobial product (disinfectants or alcohol hand sanitisers) or processes (heat) that inactivate pathogens in situ. Antimicrobials are required where adequate removal is not possible by wiping/cleaning and/or rinsing alone, or in situations of higher risk.124
Combined action, for example, laundering, where physical removal is combined with inactivation by heat together with an oxygen bleach–based laundry product.
While it is difficult to quantify the impact, evidence suggests that targeted hygiene reduces spread of infection. A review of evidence published between 1980 and 2001 concluded that the strength of the association between hygiene in the community and infections, as measured by the relative reduction in risk of illness by one or more hygiene measures (including handwashing), was generally greater than 20%.125 A meta-analysis of community studies showed that improvements in hand hygiene alone resulted in reductions in gastrointestinal and respiratory illness of 31% and 21%, respectively.126
Changing hygiene behaviour, however, requires changing public perceptions about hygiene, most particularly that hygiene is different from cleanliness, that is, more than just absence of dirt. Hygiene is what we do in the places and at the times that matter (hand, food, toilet and respiratory hygiene, health care, etc.) to protect against infection.
Communication and social marketing campaigns are now being evaluated and used as a means to achieve behaviour change mainly (but not exclusively) in relation to food and respiratory hygiene. These campaigns, however, focus on changing behaviours rather than changing understanding and dispelling misconceptions.13,127–130 The e-bug project is a Europe-wide initiative aimed at ensuring all children leave school with an understanding of targeted hygiene.131 An important feature of this teaching resource is that it is based on understanding infection and how it is transmitted.
Conclusion
The evidence reviewed in this study reflects the significant shift in thinking in the last 25 years. It shows that the interaction of the OF microbes which inhabit the natural environment and human microbiome with our immune system plays an essential role in immune regulation, promoting a tolerising milieu for the immune system which may impact against the development of allergic disease. Changes in lifestyle and environment, along with rapid urbanisation, have all contributed to changes in our exposure to essential microbes.132 In addition, altered diet and excessive antibiotic use have also sustained detrimental effects on the content and diversity of the human microbiome. Together, these factors have had profound effects on the immune system, which are likely to have contributed to the onset of allergic disease.
By contrast, the public idea that obsessive hygiene and cleanliness is the root cause of the rise in allergies is no longer supported. Data show that relevant microbial exposures are almost entirely unrelated to hygiene as the public understands it. This is partly because sustaining the human microbiome through diet and avoiding excessive antibiotic usage are factors entirely unrelated to hygiene.
As far as understanding strategies which may reduce the risk of allergic disease, work is progressing fast, but there is still a long way to go. The multiple factors involved (including those not directly associated with microbiome interactions (allergen exposure, genetic, pollution, etc.)) make it impossible to assess the contribution of each factor. It is likely that success will only be achieved through combined effects of lifestyle changes, together with improved diet and reduced antibiotic prescribing. Nevertheless, data are now strong enough to encourage changes, such as encouraging natural childbirth, physical interaction between siblings and non-siblings, more sport and other outdoor activities (including babies in prams), and less time spent indoors, and reduced antibiotic consumption.
This review further supports the view that the term ‘hygiene hypothesis’ is a misleading and dangerous misnomer which needs to be abandoned in favour of a more appropriate term such as the OF Mechanism. However, in order to tackle both allergy and infection issues we also need to develop a smarter approach to hygiene. Although targeted hygiene was developed to optimise protection against infection, it provides a framework for maximising protection against pathogen exposure but, at the same time, minimising disturbance of the indoor microbiome and spread of essential microbes between family members.
As summarised in Table 1, if we want to take advantage of these new findings, we first have to change public, public health and professional perceptions about the microbiome and about hygiene. Unstructured and conflicting advice and vague health warnings in the consumer and professional media must be replaced with simple clear mechanistic explanations and consistent messages using consistent terminology which avoids the use of the term ‘hygiene hypothesis’ to define the concept of a link between microbial exposure and allergies. Recent media articles which promote unsubstantiated suggestions that reduced handwashing could be a means to build and sustain a diverse gut microbiome are in direct conflict with public health agency advice on handwashing which is identified as probably the most important ‘critical control point’ for preventing spread of infection in all settings.133,134
An underlying problem that needs addressing is that, both nationally and internationally there are no lead agencies which take ownership of hygiene promotion, looking at it from the point of view of the public at large and what they need to understand and know. Campaigns targeting food or respiratory, pet or health-care hygiene are developed by different agencies, often with conflicting messages. They also do little to address public misunderstandings about how infections are transmitted, the difference between hygiene, cleanliness and dirt, the widespread misuse of the term ‘germs’, and the hygiene hypothesis misnomer.135
The imperative to understand and reverse the epidemiologic trends in allergic and immune-mediated disorders relates not solely to the personal suffering and health-care burden in the developed world. Without urgent effective intervention, such trends will be replicated around the globe as societies undergo socio-economic development.105
Footnotes
Funding: P.J.T. holds a Clinician Scientist Award from the UK Medical Research Council (reference MR/K010468/1) and is supported through the National Institute for Health Research (NIHR)/Imperial Biomedical Research Centre. F.S. is a founder shareholder in Atlantia Food Clinical Trials, Tucana Health, and Alimentary Health Ltd. He is director of the APC Microbiome Institute, a research centre funded in part by Science Foundation Ireland (APC/SFI/12/RC/2273) and which has recently been in receipt of research grants from the following companies: Abbvie, Alimentary Health Ltd, Cremo, Danone, General Mills, Friesland Campina, Janssen, Kerry, MeadJohnson, Nutricia, 4D Pharma plc, Second Genome, and Sigmoid pharma. The authors received an honorarium from the International Scientific Forum on Home Hygiene for their time in preparation of this manuscript.
Contributor Information
Sally F Bloomfield, London School of Hygiene & Tropical Medicine and International Scientific Forum on Home Hygiene, The Old Dairy Cottage, Montacute, Somerset TA15 6XL, UK.
Graham AW Rook, Centre for Clinical Microbiology, Department of Infection, University College London (UCL), London, UK.
Elizabeth A Scott, Center for Hygiene and Health, Department of Biology, Simmons College, Boston, MA, USA.
Fergus Shanahan, APC Microbiome Institute, University College Cork – National University of Ireland, Cork, Ireland.
Rosalind Stanwell-Smith, London School of Hygiene & Tropical Medicine, London, UK.
Paul Turner, Section of Paediatrics (Allergy & Infectious Diseases) and MRC & Asthma UK Centre in Allergic Mechanisms of Asthma, Imperial College London, London, UK; Discipline of Paediatrics and Child Health, The University of Sydney, Sydney, NSW, Australia.
References
- 1. Strachan D. Hay fever, hygiene, and household size. British Medical Journal 1989; 299: 1259–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Strachan D. Family size, infection and atopy: The first decade of the ‘hygiene hypothesis’. Thorax 2000; 55: S2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Parker W. The ‘hygiene hypothesis’ for allergic disease is a misnomer. British Medical Journal 2014; 349: g5267. [DOI] [PubMed] [Google Scholar]
- 4. Kramer A, Bekeschus S, Broker BM, Scheibinger H, Razavi B, Assadian O. Maintaining health by balancing microbial exposure and prevention of infection: The hygiene hypothesis versus the hypothesis of early immune challenge. Journal of Hospital Infection 2013; 83: S29–34. [DOI] [PubMed] [Google Scholar]
- 5. McFall-Ngai M, Hadfield MG, Bosch TC, Carey HV, Domazet-Lošo T, Douglas AE, et al. Animals in a bacterial world, a new imperative for the life sciences. Proceedings of the National Academy of Sciences U S A 2013; 110: 3229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aylon P, Ferrel WR, Stewart TR. Commentaries on ‘the Delphi technique as a forecasting tool: issues and analysis’ by Rowe and Wright. International Journal of Forecasting 1999; 15: 377–81. [Google Scholar]
- 7. Garrod B, Fyall A. Revisiting Delphi: the Delphi technique in tourism research. In: Richie BW, Burns P, Palmer C. Tourism Research Methods: Integrating Theory with Practice. Oxfordshire: Cabi Publishing, 2005, pp.85–98. [Google Scholar]
- 8. Van Teijlingen E, Pitchforth E, Bishop C, Russle E. Delphi method and nominal group technique in family planning and reproductive health technique. Journal of Family Planning and Reproductive Health 2006; 32: 249–52. [DOI] [PubMed] [Google Scholar]
- 9. Iqbal S, Pipon-Young L. The Delphi method. The Psychologist 2009; 22: 598–601. [Google Scholar]
- 10. Bloomfield SF, Exner M, Fara GM, Nath KJ, Scott EA, Van der Voorden C. The global burden of hygiene-related diseases in relation to the home and community. International Scientific Forum on Home Hygiene 2009. Available online at: http://www.ifh-homehygiene.org/review/global-burden-hygiene-related-diseases-relation-home-and-community
- 11. Parliamentary Office of Science and Technology. Food poisoning, 2003. Available online at: http://www.parliament.uk/documents/post/pn193.pdf
- 12. Tam CG, Larose T, O’Brien SJ. Costed extension to the Second Study of Infectious Intestinal Disease in the Community: Identifying the proportion of foodborne disease in the UK and attributing foodborne disease by food commodity. Food Standards Agency, 2014. Available online at: http://www.food.gov.uk
- 13. Little P, Stuart B, Hobbs FD, Moore M, Barnett J, Popoola D, et al. An internet-delivered handwashing intervention to modify influenza-like illness and respiratory infection transmission (PRIMIT): A primary care randomised trial. Lancet 2015; 386: 1631–9. [DOI] [PubMed] [Google Scholar]
- 14. Winther B, McCue K, Ashe K, Rubino J, Hendley JO. Rhinovirus contamination of surfaces in homes of adults with natural colds: Transfer of virus to fingertips during normal daily activities. Journal of Medical Virology 2011; 83: 905–9. [DOI] [PubMed] [Google Scholar]
- 15. Kirsch K. The most contaminated surfaces in hotel rooms. Presentation at the 2012 General Meeting of the American Society for Microbiology, San Francisco, CA, 16–19 June 2012. Available online at: http://www.eurekalert.org/pub_releases/2012-06/asfm-tmc061312.php [Google Scholar]
- 16. World Health Organization (WHO). Healthcare-associated infections fact sheet, 2015. Available online at: http://www.who.int/gpsc/country_work/gpsc_ccisc_fact_sheet_en.pdf
- 17. Department of Health and Health Protection Agency. Clostridium difficile infection: How to deal with the problem, 2008. Available online at: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/340851/Clostridium_difficile_infection_how_to_deal_with_the_problem.pdf
- 18. Health Protection Agency. Trends in rates of healthcare associated infection in England 2004 to 2008. Report for the National Audit Office, National Audit Office, London, June 2009. [Google Scholar]
- 19. Kings Fund. Healthcare associated infections: Stemming the rise of the ‘superbug’? 2008. Available online at: http://www.kingsfund.org.uk/sites/files/kf/briefing-healthcare-associated-infections-stemming-rise-of-superbug-rachel-turner-kings-fund-july-2008.pdf
- 20. Eyre DW, Cule ML, Wilson DJ, Griffiths D, Vaughan A, O’Connor L, et al. Diverse sources of C. difficile infection identified on whole-genome sequencing. New England Journal of Medicine 2013; 369(13): 1195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Health Protection Agency. Quarterly epidemiological commentary: Mandatory MRSA, MSSA and E. coli infection data (up to October–December 2012). Available online at: http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1284473407318
- 22. Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, et al. Global trends in emerging infectious diseases. Nature 2008; 451(7181): 990–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. World Health Organization (WHO) global influenza preparedness plan: The role of WHO and recommendations for national measures before and during pandemics. Geneva: WHO, 2005. Available online at: http://apps.who.int/iris/bitstream/10665/68998/1/WHO_CDS_CSR_GIP_2005.5.pdf [Google Scholar]
- 24. Behling RG, Eifert J, Erickson MC, Gurtler JB, Kornacki JL, Line E, et al. Selected pathogens of concern to industrial food processors: Infectious, toxigenic, toxico-infectious, selected emerging pathogenic bacteria. In Kornacki JL. (ed.) Principles of Microbiological Troubleshooting in the Industrial Food Processing Environment. New York: Springer, 2010, pp. 5–61. [Google Scholar]
- 25. Hall AJ. Noroviruses: The perfect human pathogens? Journal of Infectious Diseases 2012; 205: 1622–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Recommendations for future collaboration between the U.S. and EU. Transatlantic Taskforce on Antimicrobial Resistance, 2011. Available online at: http://ecdc.europa.eu/en/activities/diseaseprogrammes/TATFAR/Documents/210911_TATFAR_Report.pdf
- 27. Bloomfield SF. Spread of antibiotic resistant strains in the home and community. International Scientific Forum on Home Hygiene 2013. Available online at: http://www.ifh-homehygiene.org/review/spread-antibiotic-resistant-strains-home-and-community
- 28. Nachamkin I, Allos BM, Ho T. Campylobacter Species and Guillain-Barré Syndrome. Clinical Microbiology Reviews 1998; 11: 555–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yoo J, Tcheurekdjian H, Lynch SV, Cabana M, Boushey HA. Microbial manipulation of immune function for asthma prevention: Inferences from clinical trials. Proceedings of the American Thoracic Society 2007; 4: 277–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Smith V. Chapter 3. Greek hygiene. In Smith V. (ed.) Clean: A History of Personal Hygiene and Purity. Oxford: Oxford University Press, 2007, pp. 74–83. [Google Scholar]
- 31. Deckers IA, McLean S, Linssen S, Mommers M, van Schayck CP, Sheikh A. Investigating international time trends in the incidence and prevalence of atopic eczema 1990-2010: A systematic review of epidemiological studies. PLoS ONE 2012; 7(7): e39803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Asher MI, Montefort S, Björkstén B, Lai CK, Strachan DP, Weiland SK, et al.; ISAAC Phase Three Study Group. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet 2006; 368(9537): 733–43. [DOI] [PubMed] [Google Scholar]
- 33. Prescott SL, Pawankar R, Allen KJ, Campbell DE, Sinn JK, Fiocchi A, et al. A global survey of changing patterns of food allergy burden in children. World Allergy Organ Journal 2013; 6: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Platts-Mills TA. The allergy epidemics: 1870-2010. Journal of Allergy and Clinical Immunology 2015; 136: 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Venter C, Hasan Arshad S, Grundy J, Pereira B, Bernie Clayton C, Voigt K, et al. Time trends in the prevalence of peanut allergy: Three cohorts of children from the same geographical location in the UK. Allergy 2010; 65: 103–8. [DOI] [PubMed] [Google Scholar]
- 36. Perkin MR, Logan K, Tseng A, Raji B, Ayis S, Peacock J, et al. Randomized trial of introduction of allergenic foods in breast-fed infants. New England Journal of Medicine. Epub 2016. March 4. DOI: 10.1056/NEJMoa1514210. [DOI] [PubMed] [Google Scholar]
- 37. Prescott S, Allen KJ. Food allergy: Riding the second wave of the allergy epidemic. Pediatric Allergy and Immunology 2011; 22: 155–60. [DOI] [PubMed] [Google Scholar]
- 38. Rook GAW. 99th Dahlem conference on infection, inflammation and chronic inflammatory disorders: Darwinian medicine and the ‘hygiene’ or ‘old friends’ hypothesis. Clinical and Experimental Immunology 2010; 160: 70–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rook GAW. Regulation of the immune system by biodiversity from the natural environment: An ecosystem service essential to health. Proceedings of the National Academy of Science U S A 2013; 110: 18360–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rook GAW, Raison CL, Lowry CA. Microbial ‘old friends’, immunoregulation and socioeconomic status. Clinical and Experimental Immunology 2014; 177: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wolfe ND, Dunavan CP, Diamond J. Origins of major human infectious diseases. Nature 2007; 447: 279–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Benn CS, Melbye M, Wohlfahrt J, Bjorksten B, Aaby P. Cohort study of sibling effect, infectious diseases, and risk of atopic dermatitis during first 18 months of life. British Medical Journal 2004; 328: 1223–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dunder T, Tapiainen T, Pokka T, Uhari M. Infections in child day care centers and later development of asthma, allergic rhinitis, and atopic dermatitis: Prospective follow-up survey 12 years after controlled randomized hygiene intervention. Archives of Pediatric & Adolescent Medicine 2007; 161: 972–7. [DOI] [PubMed] [Google Scholar]
- 44. Bremner SA, Carey IM, DeWilde S, Richards N, Maier WC, Hilton SR, et al. Infections presenting for clinical care in early life and later risk of hay fever in two UK birth cohorts. Allergy 2008; 63: 274–83. [DOI] [PubMed] [Google Scholar]
- 45. Su LF, Kidd BA, Han A, Kotzin JJ, Davis MM. Virus-specific CD4(+) memory-phenotype T cells are abundant in unexposed adults. Immunity 2013; 38: 373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stanwell Smith R, Bloomfield SF. The hygiene hypothesis and implications for home hygiene. International Scientific Forum on Home Hygiene 2006. Available online at: http://www.ifh-homehygiene.org/best-practice-review/hygiene-hypothesis-and-implications-home-hygiene
- 47. Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Scientific Transational Medicine 2014; 6: 237–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Meropol SB, Edwards A. Development of the infant intestinal microbiome: A bird’s eye view of a complex process. Birth Defects Research Part C: Embryo Today – Reviews 2015; 105: 228–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Thavagnanam S, Fleming J, Bromley A, Shields MD, Cardwell CR. A meta-analysis of the association between Caesarean section and childhood asthma. Clinical and Experimental Allergy 2008; 38: 629–33. [DOI] [PubMed] [Google Scholar]
- 50. NHS Choices. Caesarean section, 2016. Available online at: http://www.nhs.uk/Conditions/pregnancy-and-baby/pages/caesarean-section.aspx
- 51. Jost T, Lacroix C, Braegger CP, Rochat F, Chassard C. Vertical mother-neonate transfer of maternal gut bacteria via breastfeeding. Environmental Microbiology 2013; 16: 2891–4. [DOI] [PubMed] [Google Scholar]
- 52. Penders J, Gerhold K, Stobberingh EE, Thijs MD, Zimmermann K, Lau S, et al. Establishment of the intestinal microbiota and its role for atopic dermatitis in early childhood. Journal of Allergy and Clinical Immunology 2013; 132: 601–7. [DOI] [PubMed] [Google Scholar]
- 53. Charbonneau MR, O’Donnell D, Blanton LV, Totne SM. Sialylated milk oligosaccharides promote microbiota-dependent growth in models of infant undernutrition. Cell 2016; 164: 859–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sonnenburg ED, Smits SA, Tikhonov M, Higginbottom SK, Wingreen NS, Sonnenburg JL. Diet-induced extinctions in the gut microbiota compound over generations. Nature 2016; 529: 212–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pelto GH, Zhang Y, Habicht JP. Premastication: The second arm of infant and young child feeding for health and survival? Maternal & Child Nutrition 2010; 6: 4–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hesselmar B, Sjoberg F, Saalman R, Aberg N, Adlerberth I, Wold AE. Pacifier cleaning practices and risk of allergy development. Pediatrics 2013; 131: e1829–37. [DOI] [PubMed] [Google Scholar]
- 57. Penders J, Gerhold K, Thijs C, Zimmermann K, Wahn U, Lau S, et al. New insights into the hygiene hypothesis in allergic diseases: Mediation of sibling and birth mode effects by the gut microbiota. Gut Microbes 2014; 5: 239–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ownby DR, Johnson CC, Peterson EL. Exposure to dogs and cats in the first year of life and risk of allergic sensitization at 6 to 7 years of age. JAMA 2002; 288: 963–72. [DOI] [PubMed] [Google Scholar]
- 59. Aichbhaumik N, Zoratti EM, Strickler R, Wegienka G, Owndy DR, Havstad S, et al. Exposure to household pets influences fetal immunoglobulin E production. Clinical and Experimental Allergy 2008; 38: 1787–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pet Foods Manufacturers Association, 2013. Available online at: http://www.pfma.org.uk/pet-population/
- 61. Song SJ, Lauber C, Costello EK, Lozupone CA, Humphrey G, Berg-Lyons D, et al. Cohabiting family members share microbiota with one another and with their dogs. eLife 2013; 2: e00458. Available online at: 10.7554/eLife.00458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fujimura KE, Johnson CC, Ownby DR. Man’s best friend? The effect of pet ownership on house dust microbial communities. Journal of Allergy and Clinical Immunology 2010; 126: 410–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dunn RR, Fierer N, Henley JB, Leff JW, Menninger HL. Home life: Factors structuring the bacterial diversity found within and between homes. PLoS ONE 2013; 8: e64133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Campbell BE, Lodge CJ, Lowe AJ, Burgess JA, Matheson MC, Dharmage SC. Exposure to ‘farming’ and objective markers of atopy: A systematic review and meta-analysis. Clinical and Experimental Allergy 2015; 45: 744–57. [DOI] [PubMed] [Google Scholar]
- 65. Von Mutius E, Vercelli D. Farm living: Effects on childhood asthma and allergy. Nature Reviews Immunology 2010; 10: 861–8. [DOI] [PubMed] [Google Scholar]
- 66. Kauth M, Heine H. Allergy protection by cowshed bacteria – Recent findings and future prospect. Pediatric Allergy and Immunology. Epub 2016. February 26. DOI: 10.1111/pai.12559. [DOI] [PubMed] [Google Scholar]
- 67. Ege MJ, Mayer M, Normand AC, Genuneit J, Cookson WO, Braun-friedlander C, et al. Exposure to environmental microorganisms and childhood asthma. New England Journal of Medicine 2011; 364: 701–9. [DOI] [PubMed] [Google Scholar]
- 68. Lynch SV, Wood RA, Boushey H, Bacharier LB, Bloomberg GR, Kattan M, et al. Effects of early-life exposure to allergens and bacteria on recurrent wheeze and atopy in urban children. Journal of Allergy and Clinical Immunology 2014; 134: 593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hagner S, Harb H, Zhao M, Stein K, Holst O, Ege MJ, et al. Farm-derived Gram-positive bacterium Staphylococcus sciuri W620 prevents asthma phenotype in HDM- and OVA-exposed mice. Allergy 2013; 68: 322–9. [DOI] [PubMed] [Google Scholar]
- 70. Hanski I, von Hertzen L, Fyhrquist N, Koskinen K, Torppa K, Laatikainen T, et al. Environmental biodiversity, human microbiota, and allergy are interrelated. Proceedings of the National Academy of Sciences U S A 2012; 109: 8334–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. The World Bank. Urban population, 2014. Available online at: http://data.worldbank.org/indicator/SP.URB.TOTL.IN.ZS
- 72. Indoor Air Quality UK. Available online at: http://www.iaquk.org.uk/
- 73. Schuijs MJ, Willart MA, Vergote K, Gras D, Deswarte K, Ege MJ, et al. Farm dust and endotoxin protect against allergy through A20 induction in lung epithelial cells. Science 2015; 349: 1106–10. [DOI] [PubMed] [Google Scholar]
- 74. Zhou Q, Wang H, Schwartz DM, Stoffels M, Park YH, Zhang Y, et al. Loss-of-function mutations in TNFAIP3 leading to A20 haploinsufficiency cause an early-onset autoinflammatory disease. Nature Genetics 2016; 48: 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Fyhrquist N, Ruokolainen L, Suomalainen A, Lehtimaki S, Veckman V, Vendelin J, et al. Acinetobacter species in the skin microbiota protect against allergic sensitization and inflammation. Journal of Allergy and Clinical Immunology 2014; 134: 1301–9. [DOI] [PubMed] [Google Scholar]
- 76. Hildebrand F, Nguyen TL, Brinkman B, Yunta RG, Cauwe B, Vandenabeele P, et al. Inflammation-associated enterotypes, host genotype, cage and inter-individual effects drive gut microbiota variation in common laboratory mice. Genome Biology 2013; 14: R4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. O’Toole PW, Jeffery IB. Gut microbiota and aging. Science 2015; 350: 1214–5. [DOI] [PubMed] [Google Scholar]
- 78. Claesson MJ, Jeffery IB, Conde S, Power SE, O’Connor EM, Cusack S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012; 488: 178–84. [DOI] [PubMed] [Google Scholar]
- 79. Thorburn AN, Macia L, Mackay CR. Diet, metabolites, and ‘Western-lifestyle’ inflammatory diseases. Immunity 2014; 40: 833–42. [DOI] [PubMed] [Google Scholar]
- 80. Zeng H, Chi H. Metabolic control of regulatory T cell development and function. Trends in Immunology 2015; 36: 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Martín-Peláez S, Mosele JI, Pizarro N, Farràs M, de la, Torre R, Subirana I, et al. Effect of virgin olive oil and thyme phenolic compounds on blood lipid profile: Implications of human gut microbiota. European Journal of Nutrition. Epub 2015. November 5. DOI: 10.1007/s00394-015-1063-2. [DOI] [PubMed] [Google Scholar]
- 82. Cuervo A, Hevia A, López P, Suárez A, Diaz C, Sánchez B, et al. Phenolic compounds from red wine and coffee are associated with specific intestinal microorganisms in allergic subjects. Food & Function 2016; 7: 104–9. [DOI] [PubMed] [Google Scholar]
- 83. Vanamala JK, Knight R, Spector TD. Can your microbiome tell you what to eat? Cell Metabolism 2015; 22: 960–1. [DOI] [PubMed] [Google Scholar]
- 84. Caesar R, Tremaroli V, Kovatcheva-Datchary P, Cani PD, Backhed F. Crosstalk between gut microbiota and dietary lipids aggravates WAT inflammation through TLR signaling. Cell Metabolism 2015; 22: 658–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kaliannan K, Wang B, Li XY, Kim KJ, Kang JX. A host-microbiome interaction mediates the opposing effects of omega-6 and omega-3 fatty acids on metabolic endotoxemia. Scientific Reports 2015; 5: 11276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wang M, Karlsson C, Olsson C, Adlerberth I, Wold AE, Strachan DP, et al. Reduced diversity in the early fecal microbiota of infants with atopic eczema. Journal of Allergy and Clinical Immunology 2008; 121: 129–34. [DOI] [PubMed] [Google Scholar]
- 87. Bisgaard H, Li N, Bonnelykke K, Chawes BL, Skov T, Paludan-Müller G, et al. Reduced diversity of the intestinal microbiota during infancy is associated with increased risk of allergic disease at school age. Journal of Allergy and Clinical Immunology 2011; 128: 646–52. [DOI] [PubMed] [Google Scholar]
- 88. Abrahamsson TR, Jakobsson HE, Andersson AF, Björkstén B, Engstrand L, Jenmalm MC. Low diversity of the gut microbiota in infants with atopic eczema. Journal of Allergy and Clinical Immunology 2012; 129: 434–40. [DOI] [PubMed] [Google Scholar]
- 89. Bloomfield SF. Are antibiotics a contributory factor to the rise in allergic and other chronic inflammatory diseases? International Scientific Forum on Home Hygiene 2014. Available online at: http://www.ifh-homehygiene.org/review/are-antibiotics-contributory-factor-rise-allergic-and-other-chronic-inflammatory-diseases
- 90. McKeever TM, Lewis SA, Smith C, Hubbard R. The importance of prenatal exposures on the development of allergic disease: A birth cohort study using the West Midlands General Practice Database. American Journal of Respiratory and Critical Care Medicine 2002; 166: 827–32. [DOI] [PubMed] [Google Scholar]
- 91. Metsala J, Lundqvist A, Virta LJ, Kaila M, Gissler M, Virtanen SM. Prenatal and post-natal exposure to antibiotics and risk of asthma in childhood. Clinical and Experimental Allergy 2015; 45: 137–45. [DOI] [PubMed] [Google Scholar]
- 92. Lapin B, Piorkowski J, Ownby D, Freels S, Chavez N, Hernandez E, et al. Relationship between prenatal antibiotic use and asthma in at-risk children. Annals of Allergy, Asthma & Immunology 2015; 114: 203–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Korpela K, Salonen A, Virta LJ, Kekkonen RA, Forslund K, Bork P, et al. Intestinal microbiome is related to lifetime antibiotic use in Finnish pre-school children. Nature Communications 2016; 7: 10410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Cox LM, Yamanishi S, Sohn J, Alekseyenko AV, Leung JM, Cho I, et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 2014; 158: 705–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Flores GE, Bates ST, Caporaso JG, Lauber CL, Leff JW, Knight R, et al. Diversity, distribution and sources of bacteria in residential kitchens. Environmental Microbiology 2013; 15: 588–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Scott EA, Bloomfield SF, Barlow CG. Evaluation of disinfectants in the domestic environment under ‘in use’ conditions. Journal of Hygiene 1984; 92: 193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Rusin P, Orosz-Coughlin P, Gerba C. Reduction of faecal coliform, coliform and heterotrophic plate count bacteria in the household kitchen and bathroom by disinfection with hypochlorite cleaners. Journal of Applied Microbiology 1998; 85: 819–28. [DOI] [PubMed] [Google Scholar]
- 98. Josephson KL, Rubino JR, Pepper IL. Characterization and quantification of bacterial pathogens and indicator organisms in household kitchens with and without the use of a disinfectant cleaner. Journal of Applied Microbiology 1997; 83: 737–50. [DOI] [PubMed] [Google Scholar]
- 99. Weber J, Illi S, Nowak D, Schierl R, Holst O, von Mutius E, et al. Asthma and the hygiene hypothesis. Does cleanliness matter? American Journal of Respiratory and Critical Care Medicine 2015; 191: 522–9. [DOI] [PubMed] [Google Scholar]
- 100. Liu AH. Revisiting the hygiene hypothesis for allergy and asthma. Journal of Allergy and Clinical Immunology 136; 4: 860–5. [DOI] [PubMed] [Google Scholar]
- 101. Sherriff A, Golding J. and the ALSPAC study team. Factors associated with different hygiene practices in the homes of 15 month old infants. Archives of Diseases of Childhood 2002; 86: 30–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Sherriff A, Golding J. and the ALSPAC study team. Hygiene levels in a contemporary population cohort are associated with wheezing and atopic eczema in preschool children. Archives of Diseases of Childhood 2002; 87: 26–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Hill C, Shanahan F. The enteric microbiota. In Feldman M, Friedman LS, Brandt LJ. (eds) Sleisenger and Fordtran’s Gastrointestinal and Liver Disease, 10th edn. Philadelphia, PA: Elsevier Saunders, 2015, pp. 28–35. [Google Scholar]
- 104. Shanahan F, Quigley EMM. Manipulation of the microbiota for treatment of IBS and IBD-challenges and controversies. Gastroenterology 2014; 146: 1554–63. [DOI] [PubMed] [Google Scholar]
- 105. Shanahan F. The gut microbiota – A clinical perspective on lessons learned. Nature Reviews Gastroenterology & Hepatology 2012; 9: 609–14. [DOI] [PubMed] [Google Scholar]
- 106. Shanahan F. Changing the narrative on antibiotics. Gut 2015; 64: 1674–5. [DOI] [PubMed] [Google Scholar]
- 107. Rea MC, Dobson A, O’Sullivan O, Crispie F, Fouhy F, Cotter PD, et al. Effect of broad- and narrow-spectrum antimicrobials on Clostridium difficile and microbial diversity in a model of the distal colon. Proceedings of the National Academy of Sciences U S A 2011; 108: 4639–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Franzosa EA, Huang K, Meadow JF, Gevers D, Lemon KP, Bohannan BJ, et al. Identifying personal microbiomes using metagenomic codes. Proceedings of the National Academy of Sciences U S A 2015; 112: E2930–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Zeevi D, Korem T, Zmora N, Israeli D, Rothschild D, Weinberger A, et al. Personalized nutrition by prediction of glycemic responses. Cell 2015; 163: 1079–94. [DOI] [PubMed] [Google Scholar]
- 110. Dominguez-Bello MG, De Jesus-Laboy KM, Shen N, Cox LM, Amir A, Gonzalez A, et al. Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer. Nature Medicine 2016; 22: 250–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Cunnington AJ, Sim K, Deierl A, Kroll JS, Brannigan E, Darby J. Vaginal seeding of infants born by caesarean section. British Medical Journal 2016; 352: i227. [DOI] [PubMed] [Google Scholar]
- 112. Motherway MO, Zomer A, Leahy SC, Reunanen J, Bottacini F, Claesson MJ, et al. Functional genome analysis of Bifidobacterium breve UCC2003 reveals type IVb tight adherence (Tad) pili as an essential and conserved host-colonization factor. Proceedings of the National Academy of Sciences U S A 2011; 108: 11217–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Fanning S, Hall LJ, Cronin M, Zomer A, MacSharry J, Goulding D, et al. Bifidobacterial surface-exopolysaccharide facilitates commensal-host interaction through immune modulation and pathogen protection. Proceedings of the National Academy of Sciences U S A 2012; 109: 2108–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Scott E. Foodborne disease and other hygiene issues in the home. Journal of Applied Bacteriology 1996; 80: 5–9. [DOI] [PubMed] [Google Scholar]
- 115. Nightingale F. Notes on Nursing: What It Is and What It Is Not. Glasgow; London: Blackie & Son Ltd, 1859. [Google Scholar]
- 116. Bloomfield SF, Exner M, Signorelli C, Nath KJ, Scott EA. The chain of infection transmission in the home and everyday life settings, and the role of hygiene in reducing the risk of infection. International Scientific Forum on Home Hygiene 2012. Available online at: http://www.ifh-homehygiene.org/review/chain-infection-transmissionhome-and-everyday-life-settings-and-rolehygiene-reducing-risk
- 117. Scott E. The Survival and Transfer of Pathogenic Bacteria from Environmental Sites and Surfaces. PhD thesis, Department of Pharmacy, Kings College, University of London, London, 1990. [Google Scholar]
- 118. Scott E. Community-based infections and the potential role of common touch surfaces as vectors for the transmission of infectious agents in home and community settings. American Journal of Infection Control 2013; 41: 1087–92. [DOI] [PubMed] [Google Scholar]
- 119. Cogan TA, Bloomfield SF, Humphrey TJ. The effectiveness of hygiene procedures for prevention of cross-contamination from chicken carcases in the domestic kitchen. Letters in Applied Microbiology 1999; 29: 354–8. [DOI] [PubMed] [Google Scholar]
- 120. Ramm L, Siani H, Wesgate R, Maillard J-Y. Pathogen transfer and high variability in pathogen removal by detergent wipes. American Journal of Infection Control 2015; 43: 724–8. [DOI] [PubMed] [Google Scholar]
- 121. Barker J, Vipond IB, Bloomfield SF. The effects of cleaning and disinfection in reducing the spread of Norwalk-like virus contamination via environmental surfaces. Journal of Hospital Infection 2004; 58: 42–9. [DOI] [PubMed] [Google Scholar]
- 122. Exner M, Vacata V, Hornei B, Dietlein E, Gebel J. Household cleaning and surface disinfection: New insights and strategies. Journal of Hospital Infection 2004; 56(Suppl. 2): S70–5. [DOI] [PubMed] [Google Scholar]
- 123. Sifuentes LY, Koenig DW, Philips RL, Reynolds KA, Gerba CP. Use of hygiene protocols to control the spread of viruses in a hotel. Food and Environmental Virology 2014; 6: 175–81. [DOI] [PubMed] [Google Scholar]
- 124. Bloomfield SF, Scott EA. A risk assessment approach to use of antimicrobials in the home to prevent spread of infection. American Journal of Infection Control 2013; 41: S87–93. [DOI] [PubMed] [Google Scholar]
- 125. Aiello AE, Larson EL. What is the evidence for a causal link between hygiene and infections? Lancet Infectious Disease 2002; 2: 103–10. [DOI] [PubMed] [Google Scholar]
- 126. Aiello AE, Coulborn RM, Perez V, Larson EL. Effect of hand hygiene on infectious disease risk in the community setting: A meta-analysis. American Journal of Infection Control 2008; 98: 1372–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Phillipson L, Jones SC, Larsen-Truong K, Robinson L, Barrie L. Using social marketing to promote cold and flu prevention behaviors on an Australian university campus. Cases in Public Health Communication & Marketing 2013; 7: 99–119. [Google Scholar]
- 128. FIGHTBAC The partnership for food safety education. Available online at: http://www.fightbac.org/
- 129. NHS Choices. Food safety. Available online at: http://www.nhs.uk/Livewell/homehygiene/Pages/Homehygienehub.aspx
- 130. Catch it, bin it, kill it; campaign to reduce flu infections. Available online at: https://www.gov.uk/government/news/catch-it-bin-it-kill-it-campaign-to-help-reduce-flu-infections
- 131. The e-bug project. Available online at: www.e-bug.eu
- 132. Haahtela T, Laatikainen H, Alenius P, Auvinen N, Fyhrquist I, Hanski L, et al. Hunt for the origin of allergy – Comparing the Finnish and Russian Karelia. Clinical and Experimental Allergy 2015; 45: 891–901. [DOI] [PubMed] [Google Scholar]
- 133.http://www.thetimes.co.uk/tto/health/article4602687.ece
- 134.http://www.dailymail.co.uk/sciencetech/article-3398553/Don-t-wash-hands-bit-dirt-good-Experts-say-cleaning-protect-against-allergies-allowing-helpful-bacteria-body.html#ixzz3xDe
- 135. Bloomfield SF. In future we are going to have to view our microbial world very differently. Perspectives in Public Health 2016; 136(4). [DOI] [PubMed] [Google Scholar]