Abstract
The transforming properties of oncogenes are derived from gain-of-function mutations, shifting cell signaling from highly regulated homeostatic to an uncontrolled oncogenic state, with the contribution of the inactivating mutations in tumor suppressor genes P53 and RB, leading to tumor resistance to conventional and target-directed therapy. On the other hand, this scenario fulfills two requirements for oncolytic virus infection in tumor cells: inactivation of tumor suppressors and presence of oncoproteins, also the requirements to engage malignancy. Several of these oncogenes have a negative impact on the main interferon antiviral defense, the double-stranded RNA-activated protein kinase (PKR), which helps viruses to spontaneously target tumor cells instead of normal cells. This review is focused on the negative impact of overexpression of oncogenes on conventional and targeted therapy and their positive impact on viral oncolysis due to their ability to inhibit PKR-induced translation blockage, allowing virion release and cell death.
Keywords: oncogenes, viral oncolysis, interferon, PKR, clinical trial
Introduction
Oncogenes were first identified in retroviruses and, initially, were regarded as having a retroviral origin, but further studies confirmed that these genes were captured by retroviruses from its mammalian hosts, leading to expression of altered versions of the mammalian genes.1 The transforming properties of oncogenes are derived from gain-of-function mutations, shifting from highly regulated homeostatic signaling to an uncontrolled oncogenic state.2 The most well-characterized oncogenes altered in tumors are the receptor tyrosine kinase epidermal growth factor receptor (EGFR),3 RAS,4 phosphoinositide 3-kinase (PI3K)/AKT,5 and MEK/ERK.6
Since oncogenes are part of proliferation and survival signaling pathways, their overexpression has been widely related to tumor generation, progression, and resistance to conventional chemotherapy.7 Accordingly, pharmacological inhibition of these molecules enhances chemotherapy and radiotherapy efficiency,8,9 pointing them for targeted therapy.10,11 However, the success of target-directed therapy has been challenged by the high mutation rate that alters the target, leading to development of consecutive drug generations for the same target.12
Furthermore, the inactivating mutations of the tumor suppressor genes (p53, pRB)13,14 and downregulation of proteins involved in death pathways15 also contribute to tumor resistance. This scenario, however, is extremely suitable for viral oncolysis, the lysis of a tumor cell mediated by viruses that infect and replicate inside them.16,17
Viral oncolysis
Oncolysis may be achieved by the naturally occurring oncolytic viruses, whose viral selectivity toward tumor cells is governed by the absence of factors that impair viral proliferation in the host cell (as INF type I response),18 absence of functional tumor suppressor proteins (p53 or pRb),19 and the overexpression of tumor progression factors that lead to survival signaling activation.20
On the other hand, lysis of normal cells by naturally occurring oncolytic viruses is not successful, since the host defense response, tumor suppressor, and physiological survival signaling are preserved. Additionally, these viruses themselves do not possess proteins that neutralize host defenses of normal cells. Thus, when delivered to the system, they will spontaneously target the tumor instead of normal cells.21
Few naturally occurring oncolytic viruses are available for cancer therapy. Viruses that infect human normal cells and cause disease may be modified and become suitable for viral oncolysis. The strategy involves removal of virulence factors and other genes that are not critical for the infection of tumor cells, but are vital for viral replication in normal cells, artificially creating selectivity against tumor.22
Due to this selectivity toward tumor cells, oncolytic viruses have the ability to induce cancer regression without affecting normal tissues, a feature that launched the early studies on oncolytic viruses.23,24 Viruses are obligatory intracellular parasites that depend on host cells for their propagation; thus, several viral species had evolved not only to use the host cell machinery but also to modulate main cell pathways to achieve maximum efficiency, with cancer development as a result of some viral infections.25
Even though cell surface receptors are the main feature allowing these viruses to infect the target cell,26 the way the intracellular pathways interact with the viral genome and viral proteins, which is considered vital for viral proliferation, is also important. The tumor-specific natural or genetically engineered tropism is largely based upon a defect in the type I interferon (IFN) response of many tumor cells.18,27 While in the normal tissues, IFN activation leads to inhibition of the viral replication.28
Viral oncolysis dependent on PKR inactivation
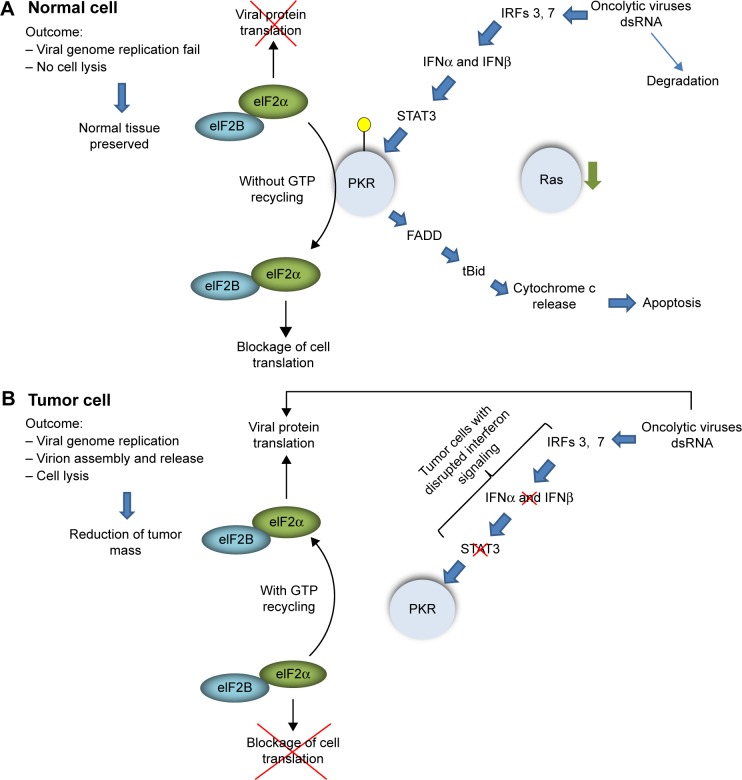
The antiviral defense system starts to act through viral nucleic acid recognition by intracellular Toll-like receptor (TLR) family. While ssRNA binds TLR-7, dsRNA binds TLR-3. The TLRs are found within the same sites that virus enters the cell.29 TLRs then induce intracellular signaling that leads to the activation of IFN regulatory factors (IRF)-3, IRF-7, and nuclear factor-kappa beta (NFκB), and the subsequent transcriptional activation of IFNα and IFNβ. Released IFNs bind to its receptors leading, through STAT3, to the transcription of the target genes, which includes PKR, the double-stranded RNA-activated protein kinase.30
PKR contains a dsRNA-binding domain that binds to duplex regions present in viral RNAs, leading to dimerization, kinase activation, and autophosphorylation of PKR.31 Activated PKR catalyze the phosphorylation of eIF2a, the translation initiation factor-2 (elF2), blocking its capacity to recycle Guanosine-5′-triphosphate (GTP). Without recycling, elF2 becomes unavailable to form the complex with Met-tRNA, impairing initiation of translation. Thus, PKR is a key mediator of the IFN type I-induced antiviral response, acting as a cytosolic sensor of viral dsRNA. In normal cells, this scenario leads to inhibition of viral genome replication and cell lysis mediated by virion release.32 Furthermore, PKR activation also induces apoptosis through FADD-mediated33 and mitochondrial pathways (Fig. 1).34
Figure 1.
Viral oncolysis dependent on RNA-activated protein kinase (PKR) inactivation. In normal cells (A), the presence of oncolytic viruses activates interferon type I pathway, leading to PKR expression and activation. Activated PKR phosphorylates elF2α, impairing GTP recycling and blocking cell translation. The presence of PKR may induce apoptosis of the infected cell. In tumor cells (B) where interferon type I response is disrupted, oncolytic viruses does not induces PKR expression, allowing GTP recycling in elF2α and viral protein translation.
Most viruses have evolved to overcome this barrier. Some carries proteins in their genomes that inhibit PKR activation through direct interaction, such as E3L and K3L from vaccinia virus,35 direct binding to PKR without activation of the kinase domain,36 and inhibition of PKR dimerization by hepatitis C virus.37 Additionally, cellular proteins can also be recruited by viruses to inhibit PKR function38 and recruitment of cellular phosphatases acting directly on elF2 phosphorylation status,39 bypassing the effects of PKR activation. On the other hand, in tumor cells, the link between INF and PKR can be disrupted by either intrinsic alteration in tumor genetics as deletion and/or loss of heterozygosis at 9p21, where INF-α, INF-β, and other INF-related genes are located,40 and overexpression of oncogenes such as RAS (Fig. 2).41
Figure 2.
The role of oncogenes in translation blockage-induced by PKR. Under overexpression of oncogenes as RAS and its downstream effectors, the interferon type I response is impaired in several points. The point mutations that make these targets resistant to therapy, maintains their activity, which affects transcription of PKR and its activation. Along with PKR inhibition, the overexpression of PI3K/Akt/mTor lead to repression of translation repressor 4E-BP1, releasing elF4E to form the Translation initiation complex, promoting viral protein translation.
Oncogene-dependent Oncolysis (Natural Tropism and/or Genetic Transformed)
The convergence of both oncogenes and some molecules encoded by viruses toward PKR is related to the basic condition for tumoral proliferation and viral infection: the maintenance or enhancement of protein synthesis rate.42 Oncolytic viruses with natural tropism find PKR inactivated in tumors due to oncogene overexpression or constitutive activation. Reovirus, a ubiquitous, nonenveloped double-stranded RNA virus, was the first naturally occurring human virus reported to exploit an oncogene signaling in the host cell to induce cell lysis.43,44 The understanding that oncogenes may spontaneously inhibit PKR launched the possibility to manipulate other viruses that normally do not cause disease in humans due to PKR activation, to infect resistant tumors. Another strategy involves the removal of PKR inhibitors carried by the viruses themselves and other virulence factors from human viruses,45 allowing them to proliferate in tumors, but not in nontumor cells. Furthermore, these viruses can also be used as vectors carrying genes that were downregulated in tumors.46
The characterization of the interaction of oncogenes with PKR showed that oncogenes have multiple roles in tumor oncolysis, and sometimes their role overlaps, with several of them present at the same signaling cascade.47 Either with inherent tropism or genetically engineered, several oncolytic pathways converge toward the RAS, PI3K, and its downstream effectors, making the oncogenes, such as RAS, RAF, MEK, ERK, and AKT, “The golden tickets” for viral oncolysis to succeed.
RAS
Physiologically, Kras, a homolog of the Kirsten murine sarcoma virus,48 and its isoforms couple activated cell surface receptors to their intracellular effector pathways. Upon binding with GTP and Guanosine diphosphate (GDP), RAS alternates between active and inactive forms, respectively. The activating mutations in RAS proteins lead to the maintenance of the GTP-bound state and the receptor-independent constitutive signaling. Upon activation, Ras recruits and activates Raf, that phosphorylates and activates MEK, which the only known substrate are ERK kinases. Active ERK translocates into the nucleus and induces phosphorylation of several targets and transcription of genes involved in cancer progression and inhibition of antiviral response.4 This modular structure makes RAS the main driver and its downstream effectors may control PKR activity either through activating their effectors downward in the cascade or acting in additional routes to allow viral oncolysis.
Ras may regulate PKR activity in several ways, including modulation of INF-induced transcription, which reduces PKR expression.49 IRF-1 has its expression modulated by MEK-downregulated IFN-inducible genes, which in turn may be negatively regulated by RAS/MEK, allowing viral oncolysis.50 Ras is important for Reovirus uncoating and infectivity.51 In modified herpes simplex virus type 1 (HSV-1), Ras signaling also dictates host–cell permissiveness.52 These and other studies are mostly based on pharmacological inhibitors of the Ras signaling pathway and cell transformation with RAS, but there is no evidence of physical interaction between RAS and PKR. Furthermore, there are few direct demonstrations of how the downstream effectors regulate the PKR activity.
RAF
Raf, the homolog of v-Raf murine sarcoma viral oncogene, the family of protein kinases (A-RAF, B-RAF, and C-RAF, also known as RAF-1), acts as a central link between Ras and the downstream kinases MEK and ERK.53,54 Activating mutations in Raf family members may render independence of Ras activation55 and resistance to targeted therapy.56 One of the most studied Raf mutations (B-RAFV600E) is also involved in viral oncolysis permissivity.57
The crucial involvement of Raf-1 as Ras cascade intermediate allowing the downregulation of JAK/STAT pathway has been shown, since knockdown of Raf-1 inhibits hepatitis C virus replication.58 Raf-1 is important not only as a part of mitogen-activated protein kinase (MAPK) cascade but also directly allows viral replication success. In parvovirus-mediated oncolysis, VP (viral capsid) proteins phosphorylation by the Raf-1 kinase at Ser-2, -6, and -10 on the VP2 N-terminal domain is essential for the nuclear translocation and capsid formation of MVM assembly intermediates.59
MEK/ERK
MEK is a key regulatory kinase activated by RAF kinases. The activating mutations in upstream members of the cascade lead to constitutive activation of MEK and its substrates, ERK1 and ERK2, in a large percentage of tumors, pointing MEK and ERK1/2 as pharmacological targets in cancer therapy.60
The resistance to MEK inhibitor is related mainly to activating mutation MEKQ60P, making it independent from RAS activation,61 and the activation of alternative activation of PI3K/AKT, leading to combination strategies, by inhibiting both MEK and AKT.62 When ERK is phosphorylated by MEK, genes involved in IFN production as IRF-1 and STAT2 are downregulated.20,50 Thus, tumors that resist to inhibitors of MEK will maintain the downregulation of PKR, allowing viral oncolysis.
Additionally, with its role in the inactivation of PKR through its downstream effectors, the importance of MEK itself for tumor oncolysis was also shown. Smith et al63 showed that activated MEK suppresses activation of PKR in mutant HSV, and they concluded that MEK could directly or indirectly inhibit PKR activity. This suggestion was reinforced by studies in vascular intimal hyperplasia using HSV-1.64
MAPKAPKs
The activation of MAPK signaling module has additional downstream players that also are able to inactivate PKR response. Among the targets of MAPKs are the MAPK-activated protein kinases (MAPKAPKs) that include MK2 and MK3. The activation of the three main cascades of the MAPK module, ERK, p38, and JNK, converge to MK2 and MK3 with ERK and p38 as their main activators.65 MK2 and MK3 possess important roles in inflammation,66 cell cycle regulation,67 and cell migration.68 In tumor cells where its upstream regulators (such as RAS and RAF) of the cascade are overexpressed, active MK2 and MK3 may interact with a complex containing repressor of the inhibitor of PKR (p88rIPK), PKR inhibitor p58IPK, and PKR itself, leading to suppression of the PKR activity and the consequent preservation of the elF2 function.69 Interestingly, overexpression of PKR inhibitor p58IPK may induce malignant transformation.70
Additional findings of this study showed that MK2 and MK3 kinases are phosphorylated and activated in lung cancer cell line under infection of influenza virus; that influenza A virus propagation is strongly reduced in mouse fibroblasts deficient in either MK2 or MK3; and that the activation of MKs reduces the activity of PKR.69
PI3K/Akt/mTOR
AKT is a serine/threonine kinase, is one of the downstream targets activated by phosphoinositide 3-kinase (PI3K), and, as other oncogenes, AKT was named after its viral oncogene homolog (v-Akt) present in the murine leukemia AKT8 retrovirus.71 Akt is a key regulator of important cellular functions, including cell survival, proliferation, glucose metabolism, and protein synthesis.72 In the majority of human cancer cells, the Akt pathway is either mutated or constitutively activated, leading to cancer progression through uncontrolled cell proliferation and apoptosis inhibition.73
The importance of AKT for viral oncolysis has been demonstrated for myxoma virus replication in human lung cancer cells, where kinase activity of Akt is required,74 while cells with no phosphorylated Akt were not infected. The same was observed in metastatic ovarian cancer that is also affected directly by AKT.75 The influence on protein synthesis by mTOR relies on regulation of translation initiation factor 4E-binding proteins (4E-BPs), promoting translation initiation.76 A pathway may be cooperatively modulated by both RAS and AKT downstream effectors.77 Sarinella et al78 found that oncolysis of pancreatic tumor by HSV-1 does not rely on RAS activation, but on PI3K in a suggested mechanism that involves modulation of elF2B through inhibition of GSK-3, leading to independence of PKR status.
Tumor suppressor protein status and PKR
The inactivating mutations found in tumor suppressors Rb and p53, associated with overexpression of RAS, may lead to tumorigenesis,79 tumor progression, and drug resistance. Among the functions performed by p53 are the cell cycle control and transcriptional activation of proapoptotic proteins, also, p53 interacts with PKR promoter, leading to its expression.80 The central role of p53 in cell death induction led several viral species to develop strategies to inhibit p53 and Rb activity in normal cells.81 In tumor cells, viral tropism is enhanced where these proteins are not functional.19
Protein Rb (retinoblastoma susceptibility protein 1) inhibits cell cycle progression from G1 to the S-phase. Under phosphorylation by cyclin D, cyclin-dependent kinase 4 (CDK4), and CDK6, Rb became inactive allowing proliferation. Overactivation of their upstream regulators, mutation, or deletion of Rb favors tumor proliferation.82
Recently, Kline et al83 showed that activation of transcription factor 4 (ATF4) in response to a small molecule required PKR activity with consequent eIF2α phosphorylation. This pathway also induced dephosphorylation of the retinoblastoma (Rb) protein, leading to inhibition of cell cycle progression. This is one example of how, under pharmacological activation, PKR and Rb contribute to tumor cell death.
Dysfunctional or deleted p53, ATM, and Rb conferred enhanced susceptibility to reovirus and myxoma viral infectivity, replication, and cytolysis in tumor cells when compared to the cells where these molecular activities are preserved.17 Modifications in adenovirus to target Rb-defective cells have been performed.84 The absence of p53 activity and the presence of activated RAS strongly reduce PKR expression and activity, along with inactivation of Rb, which sets the favorable environment for replication of oncolytic viruses.
Clinical Trials
The targeting of several oncolytic viruses under clinical trials may be spontaneous since these viruses recognize tumor cells that overexpress surface molecules such as sialic acid, CD46, and nectins, among others, which defines the tropism toward these cells.85 Viruses may also be engineered to recognize cell surface receptors of specific tumor types, such as adenovirus Ad5/3-Δ24, which was modified to bind to integrins of ovarian cancer cell surface.86 The interplay among oncogene overexpression, tumor suppressor gene status, and IFN-1 response has been explored in clinical trials using viruses that take advantage of tumor molecular alterations.
The failure to engage type I IFN signaling provides a replicative advantage for spontaneous oncolytic viruses such as Newcastle disease virus (NDV), a paramyxovirus that is low pathogenic for humans, but highly infectious in avian species. NDV is highly sensitive to type I IFN response that this virus elicits in normal cells, what confers its cancer cell specificity.87 Attenuated NDV was tested in patients with melanoma, glioblastoma, head and neck, advanced colorectal cancer, and other malignancies.88 It was used as live virus89 or NDV oncolysates.90 Other viruses of this group whose oncolytic potential were already tested clinically are Sendai virus,91 measles virus,92 and mumps virus.93 The patients generally developed flu-like symptoms and there was no death associated with the viral infection.
The production of viral RNAs by reovirus, one of the most well-studied spontaneous oncolytic virus, leads to activation of PKR in normal cells. On the other hand, overexpression of RAS impairs PKR pathway, leading to preferential infection of tumor cells by reovirus.44 Reolysin®—Reovirus Serotype-3-dearing Strain, Oncolytics Biotech Inc., a purified live replication competent reovirus, induces cytolysis in tumor cells with an activated ras pathway due to inhibition of the PKR. Reolysin administration was safe and well tolerated, showed partial response and/or stable disease in patients with breast cancer, gliomas, melanoma, and ovarian cancer,94 colorectal cancer, metastatic pancreatic adenocarcinoma, and lung cancer,95 melanoma, and head and neck sarcoma.96 On the other hand, Reolysin showed no objective responses in relapsed and refractory multiple myeloma patients.97
The importance of the tumor suppressor proteins for viral oncolysis was also explored in clinical trials. E1B is a protein that interacts with p53, allowing infection of normal cells by wild-type adenovirus.98 ONYX-015/H101 (OncorineR) is an E1B-55 kDa gene-deleted adenovirus engineered to selectively replicate in and lyse p53-deficient cancer cells.99 ONYX-015 was tested for the first time in humans in Phase I and II studies with intratumoral and peritumoral injection in 2000,100,101 leading to promising results, overall in p53-deficient cancer cells, as initially intended.
In addition to this modification, the ONYX-015 virus is “armed” with genes that enhance the immunological response toward the tumor, such as granulocyte-macrophage colony-stimulating factor (GM-CSF).22 This construction was also used in combination with mitomycin C, doxorubicin, and cisplatin adjuvant chemotherapy.102 The most common side effects were flu-like symptoms. No deaths were associated with viral infection, and no limiting dose was determined.88
The capacity of HSV-1 to induce disease in humans depends on proteins that are able to impact the INF-1 signaling, PKR activity, and tumor suppressor proteins. Therefore, important modifications were performed in order to make this virus safe for clinical use: (a) deletion of thymidine kinase (TK), a protein involved in DNA synthesis and repair,103 where expression of its cellular homolog is correlated with malignancy;104 (b) deletion of ICP 34.5 gene, which codes for a phosphatase that dephosphorylates PKR, allowing protein synthesis; and (c) deletion of ICP47, which blocks the antigen presentation in infected cells by inhibiting TAP1 and TAP2 transporters. These alterations were combined in HSV strain JS1 (JS1/34.5-/47-),105 and its clinical use has been evaluated to treat head and neck tumors and showed high rates of complete response, the absence of recurrence, and the prolonged progression-free survival seen in two-thirds of the patients strongly supporting further clinical studies.106 Equally impressive results were also achieved with unresectable metastatic melanoma.107
JX-594 (Pexa-Vec) is an oncolytic vaccinia engineered from the Wyeth vaccine strain. It has both a disruption of the TK gene and expression of human GM-CSF, which induces tumor-specific immunity.108 JX-594 was tested in hepatocellular carcinoma using systemic delivery, and the results showed a strong impact on the reduction of tumor mass and patient survival.109 JX-594 was also tested in patients with neuroblastoma and Ewing sarcoma with limited benefits.110
Taken together, the clinical trials with both attenuated and genetically modified viruses showed mostly that virotherapy success is dependent on several factors, such as administration route, with intratumoral injection showing better results over infusion due to the development of neutralizing antibody against several strains,111,112 if the virus is used as monotherapy or in combination with other drugs, or immunomodulatory strategies.108 The benefits include variable degrees of tumor remission, enhancement of free survival rate, and patient quality of life.113 Virotherapy alone was well tolerated and did not induce patient death, and the most frequent side effects were flu-like symptoms, such as fatigue and fever.100
Resistance to Viral Oncolysis
Even with its high efficiency in eliminating tumors where chemotherapy has failed, viral oncolysis also faces resistance. If several of the oncogenes that make viral oncolysis possible are resistance factors in both conventional and target-directed therapy, how resistance takes place in viral oncolysis? Maintenance of INF type I responses contributes to resistance to viral oncolysis.114 Despite the importance of RAS constitutive signaling to allow viral oncolysis, persistent infection with the reovirus led to oncolysis resistance in fibrosarcoma cells, with PKR constitutively activated, even in the presence of high Ras activity.115
Overexpression of CUG2 oncogene upregulates STAT1, leading to tumor resistance to vesicular stomatitis virus through maintenance of PKR activity.116 In addition to the PKR activity through INF type I response persistence, oncolysis resistance may occur as a result of the systemic virus clearance by the host immune system;117 the reduction of viral receptors on the target cells118 became an important barrier for viral oncolysis, once surface receptor is the main feature used by viruses to infect host cells. Survivin stabilization may lead to resistance of subpopulations of cancer cells to NDV119; and resistance to viral oncolysis may be acquired during malignant progression.120
Solid tumors are less organized in its structure and vascularity, due to defects on tumor edges and aberrant angiogenesis.121 Furthermore, extracellular matrix affect oncolysis in solid tumors, leading to the reduced virus spread.122,123 These challenges led to therapies that combine oncolytic virotherapy through intratumoral injection with antitumor conventional124 or targeted drugs,125 with promising results.
Modulation of immune response
Modulation of immune response is also an important factor in resistance to oncolysis. Preexisting or virus-induced antibodies against the oncolytic strain may be present by previous vaccination, cross-reaction, and response to oncolysis, impairing the systemic spread of the virus. An interesting example is the cross-reaction between clinically tested oncolytic Sendai virus (rodent pathogen) with human parainfluenza virus type 1 (hPIV-1).91 Thus, the vaccination history of the patient has an important impact on the therapy outcome. Additionally, in several studies where infusion was used as the administration route, the efficiency of the oncolysis was reduced overall in metastatic tumors when compared with intratumoral injection.126
One important feature of viral oncolysis is that, by killing infected tumor cells, viruses provide tumoral antigens that allow the immune system to recognize and kill more tumor cells,127 an approach that is particularly important for the treatment of metastatic lesions.128 However, the tumor microenvironment may impair the effectiveness of immune response, leading to another important source of resistance to viral oncolysis: the engagement of the immune checkpoint, a consequence of activation of a particular set of pathways that downregulate immunological efficiency against tumors.129
The programmed cell death protein 1 (PD1) and its ligand (PDL-1) may be expressed by both tumor cells and macrophages in the tumor microenvironment, turning off T-cells. The expression of PDL-1 may also occur as a consequence of translocations, chromosome aberrations, and EGFR activation,130 leading to tumor evasion. Cytotoxic T-lymphocyte antigen 4 (CTLA-4) suppresses T-cells within the lymphoid compartment by limiting T-cell proliferation, preventing expansion of antitumor T-cell responses in cancer patients.131 Immune checkpoint blockade involves the direct use of antibodies to neutralize immune checkpoint pathways (anti-PD-L1 and anti-CTLA-4),132 also, oncolytic attenuated viruses (such as measles virus) may be used as vectors to encode antibodies against CTLA-4 and PD-L1, thus enhancing the effectiveness of viral oncolysis.133
Biomarkers for viral oncolysis resistance
There are quite a few proposed markers for resistance to viral oncolysis, one of these is YAP-1 (yes-associated protein) in head and neck cancers. Its expression was correlated with resistance to reovirus, whereas low YAP-1 expression was correlated with sensitivity to reovirus infection.134 YAP-1 is a candidate human oncogene, found overexpressed in lung, colon, ovarian, and breast tumors.135 This protein is a nuclear effector of the Hippo pathway, which is a key regulator of organ size. However, this study is in its infancy, and further investigation is needed.
Concluding Remarks
The problem of conventional chemotherapy impairment by oncogene overexpression was thought to be solved when targeted therapy was introduced.136 Again, with targeted therapy, the target mutated, leading to consecutive generations of drugs to overcome resistance.137 Even though cancer virotherapy has been discussed and tested for the past 60 years,138,139 we are finally moving to what may be the golden age of virotherapy, with modified viruses with natural tropism toward tumors currently undergoing clinical trials as cancer therapeutic.94,140
The exploitation of molecular profile of tumors to achieve success in viral oncolysis highlights the importance of multidisciplinary approach that made this therapeutic alternative available. The lesson learned with in vitro and clinical experiences regarding virotherapy is that none of the previously established approaches to clinically treat cancers are dispensable. The multifactorial nature of resistance needs to be counterbalanced by multiple strategies, instead of monotherapies.
The use of multiple strategies with zero mortality involving the use of oncolytic viruses in clinical trials makes this approach efficient and safe. Although not curative yet, it certainly increases the quality and expectancy of patient’s life.109 Different from other therapeutic strategies to treat refractory tumors, in viral oncolysis, the overexpression of oncogenes mostly drive the solution, rather than the problem, partially due to INF-1 response and PKR inhibition.
Footnotes
ACADEMIC EDITOR: Barbara Guinn, Editor in Chief
PEER REVIEW: Four peer reviewers contributed to the peer review report. Reviewers’ reports totaled 641 words, excluding any confidential comments to the academic editor.
FUNDING: The author wishes to thank CNPq/INCT—Institute for Translational Research on Health and Environment in the Amazon Region—INPeTAm for financial support. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Author discloses no potential conflicts of interest.
Paper subject to independent expert single-blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: JF. Analyzed the data: JF. Wrote the first draft of the manuscript: JF. Contributed to the writing of the manuscript: JF. Agree with manuscript results and conclusions: JF. Jointly developed the structure and arguments for the paper: JF. Made critical revisions and approved final version: JF. Author reviewed and approved of the final manuscript.
REFERENCES
- 1.Vogt PK. Retroviral oncogenes: a historical primer. Nat Rev Cancer. 2012;12(9):639–648. doi: 10.1038/nrc3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohgaki H, Kleihues P. Genetic alterations and signaling pathways in the evolution of gliomas. Cancer Sci. 2009;100(12):2235–2241. doi: 10.1111/j.1349-7006.2009.01308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giaccone G, Wang Y. Strategies for overcoming resistance to EGFR family tyrosine kinase inhibitors. Cancer Treat Rev. 2011;37(6):456–464. doi: 10.1016/j.ctrv.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pylayeva-Gupta Y, Grabocka E, Bar-Sagi D. RAS oncogenes: weaving a tumorigenic web. Nat Rev Cancer. 2011;11(11):761–774. doi: 10.1038/nrc3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller TW, Rexer BN, Garrett JT, Arteaga CL. Mutations in the phosphatidylinositol 3-kinase pathway: role in tumor progression and therapeutic implications in breast cancer. Breast Cancer Res. 2011;13(6):224. doi: 10.1186/bcr3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montagut C, Settleman J. Targeting the RAF-MEK-ERK pathway in cancer therapy. Cancer Lett. 2009;283(2):125–134. doi: 10.1016/j.canlet.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 7.Zhang LL, Zhang J, Shen L, Xu XM, Yu HG. Overexpression of AKT decreases the chemosensitivity of gastric cancer cells to cisplatin in vitro and in vivo. Mol Med Rep. 2013;7(5):1387–1390. doi: 10.3892/mmr.2013.1400. [DOI] [PubMed] [Google Scholar]
- 8.Sunaga N, Shames DS, Girard L, et al. Knockdown of oncogenic KRAS in non-small cell lung cancers suppresses tumor growth and sensitizes tumor cells to targeted therapy. Mol Cancer Ther. 2011;10(2):336–346. doi: 10.1158/1535-7163.MCT-10-0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim IA, Bae SS, Fernandes A, et al. Selective inhibition of Ras, phosphoinositide 3 kinase, and akt isoforms increases the radiosensitivity of human carcinoma cell lines. Cancer Res. 2005;65(17):7902–7910. doi: 10.1158/0008-5472.CAN-05-0513. [DOI] [PubMed] [Google Scholar]
- 10.Ahmed ZA, Moatter T, Siddiqui A, Pervez S. Distribution of EGFR mutations commonly observed in primary lung adenocarcinomas in Pakistan as predictors for targeted therapy. Asian Pac J Cancer Prev. 2014;15(17):7125–7128. doi: 10.7314/apjcp.2014.15.17.7125. [DOI] [PubMed] [Google Scholar]
- 11.Wang H, Xu T, Jiang Y, et al. The challenges and the promise of molecular targeted therapy in malignant gliomas. Neoplasia. 2015;17(3):239–255. doi: 10.1016/j.neo.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandes J. The study of homology between tumor progression genes and members of retroviridae as a tool to predict target-directed therapy failure. Front Pharmacol. 2015;6:92. doi: 10.3389/fphar.2015.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vousden KH, Lu X. Live or let die: the cell’s response to p53. Nat Rev Cancer. 2002;2(8):594–604. doi: 10.1038/nrc864. [DOI] [PubMed] [Google Scholar]
- 14.Mogi A, Kuwano H. TP53 mutations in non-small cell lung cancer. J Biomed Biotechnol. 2011;2011:583929. doi: 10.1155/2011/583929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peli J, Schroter M, Rudaz C, et al. Oncogenic Ras inhibits Fas ligand-mediated apoptosis by downregulating the expression of Fas. EMBO J. 1999;18(7):1824–1831. doi: 10.1093/emboj/18.7.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cascalló M, Capellà G, Mazo A, Alemany R. Ras-dependent oncolysis with an adenovirus VAI mutant. Cancer Res. 2003;63(17):5544–5550. [PubMed] [Google Scholar]
- 17.Kim M, Williamson CT, Prudhomme J, et al. The viral tropism of two distinct oncolytic viruses, reovirus and myxoma virus, is modulated by cellular tumor suppressor gene status. Oncogene. 2010;29(27):3990–3996. doi: 10.1038/onc.2010.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zamarin D, Palese P. Oncolytic Newcastle disease virus for cancer therapy: old challenges and new directions. Future Microbiol. 2012;7(3):347–367. doi: 10.2217/fmb.12.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fasullo M, Burch AD, Britton A. Hypoxia enhances the replication of oncolytic herpes simplex virus in p53- breast cancer cells. Cell Cycle. 2009;8(14):2194–2197. doi: 10.4161/cc.8.14.8934. [DOI] [PubMed] [Google Scholar]
- 20.Christian SL, Collier TW, Zu D, Licursi M, Hough CM, Hirasawa K. Activated Ras/MEK inhibits the antiviral response of alpha interferon by reducing STAT2 levels. J Virol. 2009;83(13):6717–6726. doi: 10.1128/JVI.02213-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim M. Naturally occurring reoviruses for human cancer therapy. BMB Rep. 2015;48(8):454–460. doi: 10.5483/BMBRep.2015.48.8.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hemminki O, Parviainen S, Juhila J, et al. Immunological data from cancer patients treated with Ad5/3-E2F-Δ24-GMCSF suggests utility for tumor immunotherapy. Oncotarget. 2015;6(6):4467–4481. doi: 10.18632/oncotarget.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore AE. Viruses with oncolytic properties and their adaptation to tumors. Ann N Y Acad Sci. 1952;54:945–952. doi: 10.1111/j.1749-6632.1952.tb39969.x. [DOI] [PubMed] [Google Scholar]
- 24.Love R, Sharpless GR. Studies on a transplantable chicken tumor, RPL-12 lymphoma. II. Mechanism of regression following infection with an oncolytic virus. Cancer Res. 1954;14(9):640–647. [PubMed] [Google Scholar]
- 25.Hu D, Zhou J, Wang F, Shi H, Li Y, Li B. HPV-16 E6/E7 promotes cell migration and invasion in cervical cancer via regulating cadherin switch in vitro and in vivo. Arch Gynecol Obstet. 2015;292(6):1345–1354. doi: 10.1007/s00404-015-3787-x. [DOI] [PubMed] [Google Scholar]
- 26.Navaratnarajah CK, Miest TS, Carfi A, Cattaneo R. Targeted entry of enveloped viruses: measles and herpes simplex virus I. Curr Opin Virol. 2012;2(1):43–49. doi: 10.1016/j.coviro.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barber GN. Vesicular stomatitis virus as an oncolytic vector. Viral Immunol. 2004;17(4):516–527. doi: 10.1089/vim.2004.17.516. [DOI] [PubMed] [Google Scholar]
- 28.Schoggins JW. Interferon-stimulated genes: roles in viral pathogenesis. Curr Opin Virol. 2014;6:40–46. doi: 10.1016/j.coviro.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jensen S, Thomsen AR. Sensing of RNA viruses: a review of innate immune receptors involved in recognizing RNA virus invasion. J Virol. 2012;86(6):2900–2910. doi: 10.1128/JVI.05738-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11(5):373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 31.Husain B, Hesler S, Cole JL. Regulation of PKR by RNA: formation of active and inactive dimers. Biochemistry. 2015;54(44):6663–6672. doi: 10.1021/acs.biochem.5b01046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.García MA, Meurs EF, Esteban M. The dsRNA protein kinase PKR: virus and cell control. Biochimie. 2007;89(6–7):799–811. doi: 10.1016/j.biochi.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 33.Balachandran S, Kim CN, Yeh WC, Mak TW, Bhalla K, Barber GN. Activation of the dsRNA-dependent protein kinase, PKR, induces apoptosis through FADD-mediated death signaling. EMBO J. 1998;17(23):6888–6902. doi: 10.1093/emboj/17.23.6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gil J, Garcia MA, Esteban M. Caspase 9 activation by the dsRNA-dependent protein kinase, PKR: molecular mechanism and relevance. FEBS Lett. 2002;529(2–3):249–255. doi: 10.1016/s0014-5793(02)03348-3. [DOI] [PubMed] [Google Scholar]
- 35.Davies MV, Chang HW, Jacobs BL, Kaufman RJ. The E3L and K3L vaccinia virus gene products stimulate translation through inhibition of the double-stranded RNA-dependent protein kinase by different mechanisms. J Virol. 1993;67(3):1688–1692. doi: 10.1128/jvi.67.3.1688-1692.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Langland JO, Cameron JM, Heck MC, Jancovich JK, Jacobs BL. Inhibition of PKR by RNA and DNA viruses. Virus Res. 2006;119(1):100–110. doi: 10.1016/j.virusres.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 37.Gale M, Jr, Blakely CM, Kwieciszewski B, et al. Control of PKR protein kinase by hepatitis C virus nonstructural 5A protein: molecular mechanisms of kinase regulation. Mol Cell Biol. 1998;18(9):5208–5218. doi: 10.1128/mcb.18.9.5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharma K, Tripathi S, Ranjan P, et al. Influenza A virus nucleoprotein exploits Hsp40 to inhibit PKR activation. PLoS One. 2011;6(6):e20215. doi: 10.1371/journal.pone.0020215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kazemi S, Papadopoulou S, Li S, et al. Control of alpha subunit of eukaryotic translation initiation factor 2 (eIF2 alpha) phosphorylation by the human papillomavirus type 18 E6 oncoprotein: implications for eIF2 alpha-dependent gene expression and cell death. Mol Cell Biol. 2004;24(8):3415–3429. doi: 10.1128/MCB.24.8.3415-3429.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haus O. The genes of interferons and interferon-related factors: localization and relationships with chromosome aberrations in cancer. Arch Immunol Ther Exp (Warsz) 2000;48(2):95–100. [PubMed] [Google Scholar]
- 41.Gong J, Mita MM. Activated ras signaling pathways and reovirus oncolysis: an update on the mechanism of preferential reovirus replication in cancer cells. Front Oncol. 2014;4:167. doi: 10.3389/fonc.2014.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pervin S, Tran AH, Zekavati S, Fukuto JM, Singh R, Chaudhuri G. Increased susceptibility of breast cancer cells to stress mediated inhibition of protein synthesis. Cancer Res. 2008;68(12):4862–4874. doi: 10.1158/0008-5472.CAN-08-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strong JE, Coffey MC, Tang D, Sabinin P, Lee PW. The molecular basis of viral oncolysis: usurpation of the Ras signaling pathway by reovirus. EMBO J. 1998;17(12):3351–3362. doi: 10.1093/emboj/17.12.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marcato P, Shmulevitz M, Lee PW. Connecting reovirus oncolysis and Ras signaling. Cell Cycle. 2005;4(4):556–559. doi: 10.4161/cc.4.4.1600. [DOI] [PubMed] [Google Scholar]
- 45.Muik A, Stubbert LJ, Jahedi RZ, et al. Re-engineering vesicular stomatitis virus to abrogate neurotoxicity, circumvent humoral immunity, and enhance oncolytic potency. Cancer Res. 2014;74(13):3567–3578. doi: 10.1158/0008-5472.CAN-13-3306. [DOI] [PubMed] [Google Scholar]
- 46.Patel MR, Jacobson BA, Ji Y, et al. Vesicular stomatitis virus expressing interferon-β is oncolytic and promotes antitumor immune responses in a syngeneic murine model of non-small cell lung cancer. Oncotarget. 2015;6(32):33165–33177. doi: 10.18632/oncotarget.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Toulany M, Minjgee M, Saki M, et al. ERK2-dependent reactivation of Akt mediates the limited response of tumor cells with constitutive K-RAS activity to PI3K inhibition. Cancer Biol Ther. 2014;15(3):317–328. doi: 10.4161/cbt.27311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aaronson SA, Weaver CA. Characterization of murine sarcoma virus (Kirsten) transformation of mouse and human cells. J Gen Virol. 1971;13(2):245–252. doi: 10.1099/0022-1317-13-2-245. [DOI] [PubMed] [Google Scholar]
- 49.Christian SL, Zu D, Licursi M, et al. Suppression of IFN-induced transcription underlies IFN defects generated by activated Ras/MEK in human cancer cells. PLoS One. 2012;7(9):e44267. doi: 10.1371/journal.pone.0044267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Komatsu Y, Christian SL, Ho N, Pongnopparat T, Licursi M, Hirasawa K. Oncogenic Ras inhibits IRF1 to promote viral oncolysis. Oncogene. 2015;34(30):3985–3993. doi: 10.1038/onc.2014.331. [DOI] [PubMed] [Google Scholar]
- 51.Marcato P, Shmulevitz M, Pan D, Stoltz D, Lee PW. Ras transformation mediates reovirus oncolysis by enhancing virus uncoating, particle infectivity, and apoptosis-dependent release. Mol Ther. 2007;15(8):1522–1530. doi: 10.1038/sj.mt.6300179. [DOI] [PubMed] [Google Scholar]
- 52.Farassati F, Yang AD, Lee PW. Oncogenes in Ras signalling pathway dictate host-cell permissiveness to herpes simplex virus 1. Nat Cell Biol. 2001;3(8):745–750. doi: 10.1038/35087061. [DOI] [PubMed] [Google Scholar]
- 53.Wellbrock C, Karasarides M, Marais R. The RAF proteins take centre stage. Nat Rev Mol Cell Biol. 2004;5(11):875–885. doi: 10.1038/nrm1498. [DOI] [PubMed] [Google Scholar]
- 54.Freeman AK, Ritt DA, Morrison DK. The importance of Raf dimerization in cell signaling. Small GTPases. 2013;4(3):180–185. doi: 10.4161/sgtp.26117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 56.Sievert AJ, Lang SS, Boucher KL, et al. Paradoxical activation and RAF inhibitor resistance of BRAF protein kinase fusions characterizing pediatric astrocytomas. Proc Natl Acad Sci U S A. 2013;110(15):5957–5962. doi: 10.1073/pnas.1219232110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wollmann G, Davis JN, Bosenberg MW, van den Pol AN. Vesicular stomatitis virus variants selectively infect and kill human melanomas but not normal melanocytes. J Virol. 2013;87(12):6644–6659. doi: 10.1128/JVI.03311-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Q, Gong R, Qu J, et al. Activation of the Ras/Raf/MEK pathway facilitates hepatitis C virus replication via attenuation of the interferon-JAK-STAT pathway. J Virol. 2012;86(3):1544–1554. doi: 10.1128/JVI.00688-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Riolobos L, Valle N, Hernando E, Maroto B, Kann M, Almendral JM. Viral oncolysis that targets Raf-1 signaling control of nuclear transport. J Virol. 2010;84(4):2090–2099. doi: 10.1128/JVI.01550-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ryan MB, Der CJ, Wang-Gillam A, Cox AD. Targeting RAS-mutant cancers: is ERK the key? Trends Cancer. 2015;1(3):183–198. doi: 10.1016/j.trecan.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wagle N, Van Allen EM, Treacy DJ, et al. MAP kinase pathway alterations in BRAF-mutant melanoma patients with acquired resistance to combined RAF/MEK inhibition. Cancer Discov. 2014;4(1):61–68. doi: 10.1158/2159-8290.CD-13-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Park KS, Oh B, Lee MH, et al. The HSP90 inhibitor, NVP-AUY922, sensitizes KRAS-mutant non-small cell lung cancer with intrinsic resistance to MEK inhibitor, trametinib. Cancer Lett. 2016;372(1):75–81. doi: 10.1016/j.canlet.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 63.Smith KD, Mezhir JJ, Bickenbach K, et al. Activated MEK suppresses activation of PKR and enables efficient replication and in vivo oncolysis by Delta-gamma(1)34.5 mutants of herpes simplex virus 1. J Virol. 2006;80(3):1110–1120. doi: 10.1128/JVI.80.3.1110-1120.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Skelly CL, He Q, Spiguel L, McCormick S, Weichselbaum R. Modulating vascular intimal hyperplasia using HSV-1 mutant requires activated MEK. Gene Ther. 2013;20(2):215–224. doi: 10.1038/gt.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev. 2004;68(2):320–344. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moens U, Kostenko S, Sveinbjørnsson B. The role of mitogen-activated protein kinase-activated protein kinases (MAPKAPKs) in inflammation. Genes (Basel) 2013;4(2):101–133. doi: 10.3390/genes4020101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Prickaerts P, Niessen HE, Dahlmans VE, et al. MK3 modulation affects BMI1-dependent and independent cell cycle check-points. PLoS One. 2015;10(4):e0118840. doi: 10.1371/journal.pone.0118840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gamell C, Susperregui AG, Bernard O, Rosa JL, Ventura F. The p38/MK2/Hsp25 pathway is required for BMP-2-induced cell migration. PLoS One. 2011;6(1):e16477. doi: 10.1371/journal.pone.0016477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Luig C, Köther K, Dudek SE, et al. MAP kinase-activated protein kinases 2 and 3 are required for influenza A virus propagation and act via inhibition of PKR. FASEB J. 2010;24(10):4068–4077. doi: 10.1096/fj.10-158766. [DOI] [PubMed] [Google Scholar]
- 70.Barber GN, Thompson S, Lee TG, et al. The 58-kilodalton inhibitor of the interferon-induced double-stranded RNA-activated protein kinase is a tetratricopeptide repeat protein with oncogenic properties. Proc Natl Acad Sci U S A. 1994;91(10):4278–4282. doi: 10.1073/pnas.91.10.4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Staal SP. Molecular cloning of the akt oncogene and its human homologues AKTI and AKT2: amplification of AKTI in a primary human gastric adenocarcinoma. Proc Natl Acad Sci U S A. 1987;84(14):5034–5037. doi: 10.1073/pnas.84.14.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Martelli AM, Tazzari PL, Evangelisti C, et al. Targeting the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin module for acute myelogenous leukemia therapy: from bench to bedside. Curr Med Chem. 2007;14(19):2009–2023. doi: 10.2174/092986707781368423. [DOI] [PubMed] [Google Scholar]
- 73.Brognard J, Clark AS, Ni Y, Dennis PA. Akt/protein kinase B is constitutively active in non-small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation. Cancer Res. 2001;61(10):3986–3997. [PubMed] [Google Scholar]
- 74.Werden SJ, McFadden G. Pharmacological manipulation of the akt signaling pathway regulates myxoma virus replication and tropism in human cancer cells. J Virol. 2010;84(7):3287–3302. doi: 10.1128/JVI.02020-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Correa RJ, Komar M, Tong JG, et al. Myxoma virus-mediated oncolysis of ascites-derived human ovarian cancer cells and spheroids is impacted by differential AKT activity. Gynecol Oncol. 2012;125(2):441–450. doi: 10.1016/j.ygyno.2012.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gingras AC, Kennedy SG, O’Leary MA, Sonenberg N, Hay N. 4E-BP1, a repressor of mRNA translation, is phosphorylated and inactivated by the Akt(PKB) signaling pathway. Genes Dev. 1998;12(4):502–513. doi: 10.1101/gad.12.4.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.She QB, Halilovic E, Ye Q, et al. 4E-BP1 is a key effector of the oncogenic activation of the AKT and ERK signaling pathways that integrates their function in tumors. Cancer Cell. 2010;18(1):39–51. doi: 10.1016/j.ccr.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sarinella F, Calistri A, Sette P, Palù G, Parolin C. Oncolysis of pancreatic tumour cells by a gamma34.5-deleted HSV-1 does not rely upon Ras-activation, but on the PI 3-kinase pathway. Gene Ther. 2006;13(14):1080–1087. doi: 10.1038/sj.gt.3302770. [DOI] [PubMed] [Google Scholar]
- 79.Kendall SD, Linardic CM, Adam SJ, Counter CM. A network of genetic events sufficient to convert normal human cells to a tumorigenic state. Cancer Res. 2005;65(21):9824–9828. doi: 10.1158/0008-5472.CAN-05-1543. [DOI] [PubMed] [Google Scholar]
- 80.Yoon CH, Lee ES, Lim DS, Bae YS. PKR, a p53 target gene, plays a crucial role in the tumor-suppressor function of p53. Proc Natl Acad Sci U S A. 2009;106(19):7852–7857. doi: 10.1073/pnas.0812148106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ruttkay-Nedecky B, Jimenez Jimenez AM, Nejdl L, et al. Relevance of infection with human papillomavirus: the role of the p53 tumor suppressor protein and E6/E7 zinc finger proteins (Review) Int J Oncol. 2013;43(6):1754–1762. doi: 10.3892/ijo.2013.2105. [DOI] [PubMed] [Google Scholar]
- 82.Rutka JT, Akiyama Y, Lee SP, Ivanchuk S, Tsugu A, Hamel PA. Alterations of the p53 and pRB pathways in human astrocytoma. Brain Tumor Pathol. 2000;17(2):65–70. doi: 10.1007/BF02482737. [DOI] [PubMed] [Google Scholar]
- 83.Kline CL, Van den Heuvel AP, Allen JE, Prabhu VV, Dicker DT, El-Deiry WS. ONC201 kills solid tumor cells by triggering an integrated stress response dependent on ATF4 activation by specific eIF2α kinases. Sci Signal. 2016;9(415):ra18. doi: 10.1126/scisignal.aac4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jakubczak JL, Ryan P, Gorziglia M, et al. An oncolytic adenovirus selective for retinoblastoma tumor suppressor protein pathway-defective tumors: dependence on E1A, the E2F-1 promoter, and viral replication for selectivity and efficacy. Cancer Res. 2003;63(7):1490–1499. [PubMed] [Google Scholar]
- 85.Noyce RS, Richardson CD. Nectin 4 is the epithelial cell receptor for measles virus. Trends Microbiol. 2012;20(9):429–439. doi: 10.1016/j.tim.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 86.Kanerva A, Mikheeva GV, Krasnykh V, et al. Targeting adenovirus to the serotype 3 receptor increases gene transfer efficiency to ovarian cancer cells. Clin Cancer Res. 2002;8(1):275–280. [PubMed] [Google Scholar]
- 87.Park MS, Shaw ML, Muñoz-Jordan J, et al. Newcastle disease virus (NDV)-based assay demonstrates interferon-antagonist activity for the NDV V protein and the Nipah virus V, W, and C proteins. J Virol. 2003;77(2):1501–1511. doi: 10.1128/JVI.77.2.1501-1511.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Matveeva OV, Guo ZS, Senin VM, Senina AV, Shabalina SA, Chumakov PM. Oncolysis by paramyxoviruses: preclinical and clinical studies. Mol Ther Oncolytics. 2015;2:ii:150017. doi: 10.1038/mto.2015.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Csatary LK, Gosztonyi G, Szeberenyi J, et al. MTH-68/H oncolytic viral treatment in human high-grade gliomas. J Neurooncol. 2004;67(1–2):83–93. doi: 10.1023/b:neon.0000021735.85511.05. [DOI] [PubMed] [Google Scholar]
- 90.Batliwalla FM, Bateman BA, Serrano D, et al. A 15-year follow-up of AJCC stage III malignant melanoma patients treated postsurgically with Newcastle disease virus (NDV) oncolysate and determination of alterations in the CD8 T cell repertoire. Mol Med. 1998;4(12):783–794. [PMC free article] [PubMed] [Google Scholar]
- 91.Slobod KS, Shenep JL, Luján-Zilbermann J, et al. Safety and immunogenicity of intranasal murine parainfluenza virus type 1 (Sendai virus) in healthy human adults. Vaccine. 2004;22(23–24):3182–3186. doi: 10.1016/j.vaccine.2004.01.053. [DOI] [PubMed] [Google Scholar]
- 92.Galanis E, Hartmann LC, Cliby WA, et al. Phase I trial of intraperitoneal administration of an oncolytic measles virus strain engineered to express carcinoembryonic antigen for recurrent ovarian cancer. Cancer Res. 2010;70(3):875–882. doi: 10.1158/0008-5472.CAN-09-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Asada T. Treatment of human cancer with mumps virus. Cancer. 1974;34(6):1907–1928. doi: 10.1002/1097-0142(197412)34:6<1907::aid-cncr2820340609>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 94.Gollamudi R, Ghalib MH, Desai KK, et al. Intravenous administration of Reolysin, a live replication competent RNA virus is safe in patients with advanced solid tumors. Invest New Drugs. 2010;28(5):641–649. doi: 10.1007/s10637-009-9279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Villalona-Calero MA, Lam E, Otterson GA, et al. Oncolytic reovirus in combination with chemotherapy in metastatic or recurrent non-small cell lung cancer patients with KRAS-activated tumors. Cancer. 2016;122(6):875–883. doi: 10.1002/cncr.29856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Morris DG, Feng X, DiFrancesco LM, et al. REO-001: A phase I trial of percutaneous intralesional administration of reovirus type 3 dearing (Reolysin®) in patients with advanced solid tumors. Invest New Drugs. 2013;31(3):696–706. doi: 10.1007/s10637-012-9865-z. [DOI] [PubMed] [Google Scholar]
- 97.Sborov DW, Nuovo GJ, Stiff A, et al. A phase I trial of single-agent reolysin in patients with relapsed multiple myeloma. Clin Cancer Res. 2014;20(23):5946–5955. doi: 10.1158/1078-0432.CCR-14-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Roth J, Dobbelstein M. Interaction of p53 with the adenovirus E1B-55 kDa protein. Methods Mol Biol. 2003;234:135–149. doi: 10.1385/1-59259-408-5:135. [DOI] [PubMed] [Google Scholar]
- 99.McCormick F. Interactions between adenovirus proteins and the p53 pathway: the development of ONYX-015. Semin Cancer Biol. 2000;10(6):453–459. doi: 10.1006/scbi.2000.0336. [DOI] [PubMed] [Google Scholar]
- 100.Ganly I, Kirn D, Eckhardt G, et al. A phase I study of Onyx-015, an E1B attenuated adenovirus, administered intratumorally to patients with recurrent head and neck cancer. Clin Cancer Res. 2000;6(3):798–806. [PubMed] [Google Scholar]
- 101.Nemunaitis J, Ganly I, Khuri F, et al. Selective replication and oncolysis in p53 mutant tumors with ONYX-015, an E1B-55kD gene-deleted adenovirus, in patients with advanced head and neck cancer: a phase II trial. Cancer Res. 2000;60(22):6359–6366. [PubMed] [Google Scholar]
- 102.Opyrchal M, Aderca I, Galanis E. Phase I clinical trial of locoregional administration of the oncolytic adenovirus ONYX-015 in combination with mitomycin-C, doxorubicin, and cisplatin chemotherapy in patients with advanced sarcomas. Methods Mol Biol. 2009;542:705–717. doi: 10.1007/978-1-59745-561-9_35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hengstschläger M, Pfeilstöcker M, Wawra E. Thymidine kinase expression. A marker for malignant cells. Adv Exp Med Biol. 1998;431:455–460. [PubMed] [Google Scholar]
- 104.Alegre MM, Robison RA, O’Neill KL. Thymidine kinase 1 upregulation is an early event in breast tumor formation. J Oncol. 2012;2012:575647. doi: 10.1155/2012/575647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu BL, Robinson M, Han ZQ, et al. ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Ther. 2003;10(4):292–303. doi: 10.1038/sj.gt.3301885. [DOI] [PubMed] [Google Scholar]
- 106.Harrington KJ, Hingorani M, Tanay MA, et al. Phase I/II study of oncolytic HSV GM-CSF in combination with radiotherapy and cisplatin in untreated stage III/IV squamous cell cancer of the head and neck. Clin Cancer Res. 2010;16(15):4005–4015. doi: 10.1158/1078-0432.CCR-10-0196. [DOI] [PubMed] [Google Scholar]
- 107.Senzer NN, Kaufman HL, Amatruda T, et al. Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J Clin Oncol. 2009;27(34):5763–5771. doi: 10.1200/JCO.2009.24.3675. [DOI] [PubMed] [Google Scholar]
- 108.Mastrangelo MJ, Maguire HC, Jr, Eisenlohr LC, et al. Intratumoral recombinant GM-CSF-encoding virus as gene therapy in patients with cutaneous melanoma. Cancer Gene Ther. 1999;6(5):409–422. doi: 10.1038/sj.cgt.7700066. [DOI] [PubMed] [Google Scholar]
- 109.Heo J, Reid T, Ruo L, et al. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat Med. 2013;19(3):329–336. doi: 10.1038/nm.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cripe TP, Ngo MC, Geller JI, et al. Phase 1 study of intratumoral Pexa-Vec (JX-594), an oncolytic and immunotherapeutic vaccinia virus, in pediatric cancer patients. Mol Ther. 2015;23(3):602–608. doi: 10.1038/mt.2014.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Miest TS, Yaiw KC, Frenzke M, et al. Envelope-chimeric entry-targeted measles virus escapes neutralization and achieves oncolysis. Mol Ther. 2011;19(10):1813–1820. doi: 10.1038/mt.2011.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jennings VA, Ilett EJ, Scott KJ, et al. Lymphokine-activated killer and dendritic cell carriage enhances oncolytic reovirus therapy for ovarian cancer by overcoming antibody neutralization in ascites. Int J Cancer. 2014;134(5):1091–1101. doi: 10.1002/ijc.28450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gong J, Sachdev E, Mita AC, Mita MM. Clinical development of reovirus for cancer therapy: an oncolytic virus with immune-mediated antitumor activity. World J Methodol. 2016;6(1):25–42. doi: 10.5662/wjm.v6.i1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Moerdyk-Schauwecker M, Shah NR, Murphy AM, Hastie E, Mukherjee P, Grdzelishvili VZ. Resistance of pancreatic cancer cells to oncolytic vesicular stomatitis virus: role of type I interferon signaling. Virology. 2013;436(1):221–234. doi: 10.1016/j.virol.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kim M, Egan C, Alain T, et al. Acquired resistance to reoviral oncolysis in Ras-transformed fibrosarcoma cells. Oncogene. 2007;26(28):4124–4134. doi: 10.1038/sj.onc.1210189. [DOI] [PubMed] [Google Scholar]
- 116.Malilas W, Koh SS, Srisuttee R, et al. Cancer upregulated gene 2, a novel oncogene, confers resistance to oncolytic vesicular stomatitis virus through STAT1-OASL2 signaling. Cancer Gene Ther. 2013;20(2):125–132. doi: 10.1038/cgt.2012.96. [DOI] [PubMed] [Google Scholar]
- 117.Thomas MA, Spencer JF, Toth K, Sagartz JE, Phillips NJ, Wold WS. Immunosuppression enhances oncolytic adenovirus replication and antitumor efficacy in the Syrian hamster model. Mol Ther. 2008;16(10):1665–1673. doi: 10.1038/mt.2008.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Strauss R, Sova P, Liu Y, et al. Epithelial phenotype confers resistance of ovarian cancer cells to oncolytic adenoviruses. Cancer Res. 2009;69(12):5115–5125. doi: 10.1158/0008-5472.CAN-09-0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jamal MH, Ch’ng WC, Yusoff K, Shafee N. Reduced Newcastle disease virus-induced oncolysis in a subpopulation of cisplatin-resistant MCF7 cells is associated with survivin stabilization. Cancer Cell Int. 2012;12(1):35. doi: 10.1186/1475-2867-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yu N, Puckett S, Antinozzi PA, Cramer SD, Lyles DS. Changes in susceptibility to oncolytic vesicular stomatitis virus during progression of prostate cancer. J Virol. 2015;89(10):5250–5263. doi: 10.1128/JVI.00257-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Choi IK, Strauss R, Richter M, Yun CO, Lieber A. Strategies to increase drug penetration in solid tumors. Front Oncol. 2013;3:193. doi: 10.3389/fonc.2013.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Smith E, Breznik J, Lichty BD. Strategies to enhance viral penetration of solid tumors. Hum Gene Ther. 2011;22(9):1053–1060. doi: 10.1089/hum.2010.227. [DOI] [PubMed] [Google Scholar]
- 123.Yaacov B, Lazar I, Tayeb S, et al. Extracellular matrix constituents interfere with Newcastle disease virus spread in solid tissue and diminish its potential oncolytic activity. J Gen Virol. 2012;93(pt 8):1664–1672. doi: 10.1099/vir.0.043281-0. [DOI] [PubMed] [Google Scholar]
- 124.Gomez-Gutierrez JG, Nitz J, Sharma R, et al. Combined therapy of oncolytic adenovirus and temozolomide enhances lung cancer virotherapy in vitro and in vivo. Virology. 2016;487:249–259. doi: 10.1016/j.virol.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Marchini A, Scott EM, Rommelaere J. Overcoming barriers in oncolytic virotherapy with HDAC inhibitors and immune checkpoint blockade. Viruses. 2016;8(1):ii:E9. doi: 10.3390/v8010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kim YI, Ahn BC, Ronald JA, et al. Intratumoral versus intravenous gene therapy using a transcriptionally targeted viral vector in an orthotopic hepatocellular carcinoma rat model. J Vasc Interv Radiol. 2012;23(5):704–711. doi: 10.1016/j.jvir.2012.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tong AW, Senzer N, Cerullo V, Templeton NS, Hemminki A, Nemunaitis J. Oncolytic viruses for induction of anti-tumor immunity. Curr Pharm Biotechnol. 2012;13(9):1750–1760. doi: 10.2174/138920112800958913. [DOI] [PubMed] [Google Scholar]
- 128.Hwang TH, Moon A, Burke J, et al. A mechanistic proof-of-concept clinical trial with JX-594, a targeted multi-mechanistic oncolytic poxvirus, in patients with metastatic melanoma. Mol Ther. 2011;19(10):1913–1922. doi: 10.1038/mt.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Minn AJ, Wherry EJ. Combination cancer therapies with immune checkpoint blockade: convergence on interferon signaling. Cell. 2016;165(2):272–275. doi: 10.1016/j.cell.2016.03.031. [DOI] [PubMed] [Google Scholar]
- 130.Rech AJ, Vonderheide RH. Dynamic interplay of oncogenes and T cells induces PD-L1 in the tumor microenvironment. Cancer Discov. 2013;3(12):1330–1332. doi: 10.1158/2159-8290.CD-13-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271(5256):1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 132.Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16(5):275–287. doi: 10.1038/nrc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Engeland CE, Grossardt C, Veinalde R, et al. CTLA-4 and PD-L1 checkpoint blockade enhances oncolytic measles virus therapy. Mol Ther. 2014;22(11):1949–1959. doi: 10.1038/mt.2014.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bolton GC, Simpson GR, Coffey M, et al. Resistance to oncolytic reovirus is associated with high expression of yes-associated protein-1 (YAP-1) in head and neck cancer. Hum Gene Ther. 2014;25(12):A12. [Google Scholar]
- 135.Steinhardt AA, Gayyed MF, Klein AP, et al. Expression of yes-associated protein in common solid tumors. Hum Pathol. 2008;39(11):1582–1589. doi: 10.1016/j.humpath.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Neal JW, Sledge GW. Decade in review-targeted therapy: successes, toxicities and challenges in solid tumours. Nat Rev Clin Oncol. 2014;11(11):627–628. doi: 10.1038/nrclinonc.2014.171. [DOI] [PubMed] [Google Scholar]
- 137.Eberlein CA, Stetson D, Markovets AA, et al. Acquired resistance to the mutant-selective EGFR inhibitor AZD9291 is associated with increased dependence on RAS signaling in preclinical models. Cancer Res. 2015;75(12):2489–2500. doi: 10.1158/0008-5472.CAN-14-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Southam CM, Babcock VI. Viral oncolysis studies with a metastatic human tumor in chicks. Cancer Res. 1958;18(9):1070–1074. [PubMed] [Google Scholar]
- 139.Cassel WA, Garrett RE. Tumor immunity after viral oncolysis. J Bacteriol. 1966;92(3):792. doi: 10.1128/jb.92.3.792-.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Galanis E, Markovic SN, Suman VJ, et al. Phase II trial of intravenous administration of Reolysin(®) (reovirus serotype-3-dearing strain) in patients with metastatic melanoma. Mol Ther. 2012;20(10):1998–2003. doi: 10.1038/mt.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]