Table 15.
Details of different anti-HER2 ADCs in development
| ADC name | Antibody | Linker | Toxin | Key IP right | Company | Status | Claim language |
|---|---|---|---|---|---|---|---|
| Trastuzumab emtansine | Trastuzumab | SMCC | DM1 | US8337856 | Genentech | Approved in US and EU | An immunoconjugate comprising an anti-ErbB2 antibody conjugated to a maytansinoid, wherein the antibody is huMAb4D5–8. |
| MM-302 | scFv anti-HER2 | PEG-DSPE | Liposome-encapsulateddoxorubicin | US2014023698 | Merrimack | Phase 2/3 | A method of treating a human cancer patient by administration of anthracycline - comprising anti-HER2 immunoliposomes, the method comprising determining a first dosage, such a dosage indicating a dose magnitude and frequency of dosing, for a patient diagnosed with a cancer characterized by expression of HER2 receptor, the first dosage being for a liposomal anthracycline chemotherapeutic agent that does not comprise an immunoliposome, which dosage is determined to provide to the patient a safe and effective amount of the liposomal formulation, and administering anthracycline-comprising anti-HER2 immunoliposomes, a plurality of which immunoliposomes is each bearing a plurality of anti-HER2 antibody molecules on its surface and each containing the anthracycline chemotherapeutic agent, wherein the anthracycline-comprising anti-HER2 immunoliposomes are administered to the patient at the first dosage. |
| SYD985 | Trastuzumab | SpaceLink | Duocarmycin | EP2560645A2 | Syntarga | Phase 1 | A compound of formula (III):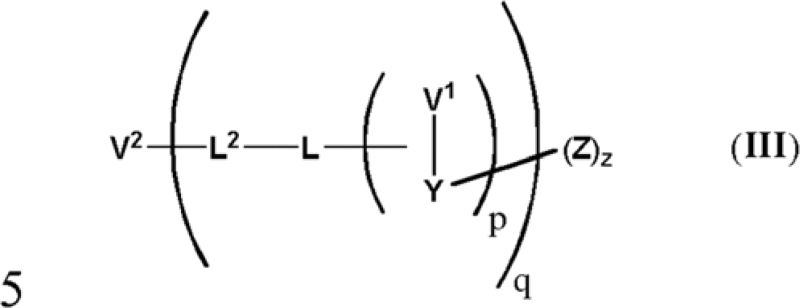 or a pharmaceutically acceptable salt, hydrate, or solvate thereof, wherein […] |
| ARX788 | Undisclosed | Undisclosed | Amberstatin AS269 | US2015141624 | AmbrX | Preclinical* |
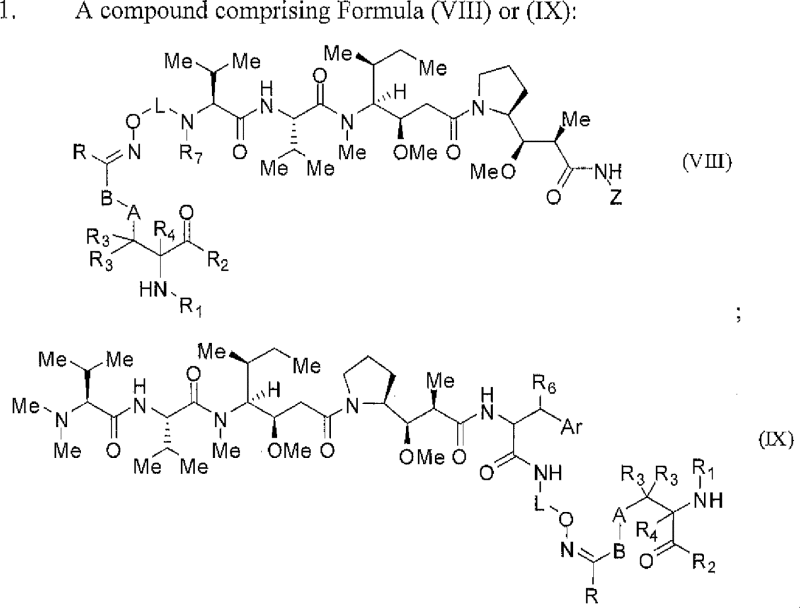 wherein […] |
| Not available | Trastuzumab | Transglutaminase + EENC linker | MMAE, hybrid ADCs with different toxins | Not available | Dophen | R&D | Not available |
| Not available | Trastuzumab | SMAC with N-terminal LPTXG tag/linker | (i) DM1 (mertansine), (ii) maytansine, (iii) MMAE/MMAF (iv) α-amanitin (v) undisclosed new toxin, respectively |
(i) – (iv) WO2014140317 (v) not disclosed |
NBE Therapeutics | R&D | 1. A method of producing an immunoligand/payload conjugate, which method encompasses conjugating a payload to an immunoligand by means of a sequence- specific transpeptidase, or a catalytic domain thereof. 6. The method according to any of the aforementioned claims, wherein the sequence-specific transpepeptidase is at least one selected from the group consisting of• a sortase enzyme, or one or more fragments or derivatives thereof• a split-intein, or one or more fragments or derivatives thereof |
| Not available | Trastuzumab | N-Hydroxysuccinimide ester | Tubulysin | Not available | VUMC Amsterdam | R&D | Not available |
Phase 1 study (NCT02512237) scheduled to start in September 2015; the symbol […] indicates that claim language has been truncated.
