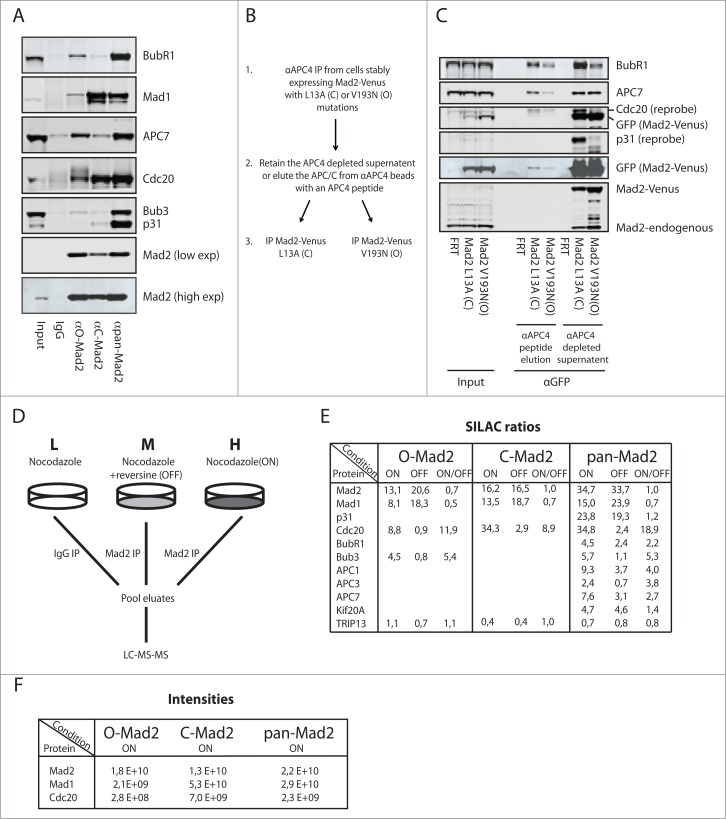
Figure 2.
Analysis of Mad2 complexes using conformation specific antibodies. (A) HeLa cells arrested in mitosis with nocodazole were harvested by mitotic shake-off and Mad2 complexes purified with the different Mad2 monoclonal antibodies. Representative of 3 independent experiments. (B) Schematic of the sequential purification of Mad2-Venus complexes. (C) HeLa FRT/Trex cells stably expressing Mad2 L13A-Venus or Mad2 V193N-Venus were collected by mitotic shake-off after nocodazole treatment. APC/C complexes were purified with a monoclonal APC4 antibody and then eluted by the antigenic peptide. The eluate containing APC/C and the supernatant after APC/C depletion was then used for purification of Mad2-Venus complexes using GFP-Trap beads (chromotek) and analyzed by protein gel blot. Input is total cell extract prior to APC4 purification. Representative of 2 independent experiments. (D) Schematic of the triple SILAC experimental set-up. L: light condition, M: medium condition, H: heavy condition. (E) SILAC ratios determined for known Mad2 interactors in the SAC ON and SAC OFF condition. Only known interactors having a SILAC ratio higher than 2 are included except for TRIP13, which is included despite not being enriched above IgG control levels. (F) Peptide intensities for Mad2, Mad1 and Cdc20 in the indicated conditions to illustrate that the O-Mad2 antibody purifies much lower levels of Mad1 and Cdc20.