Figure 5.
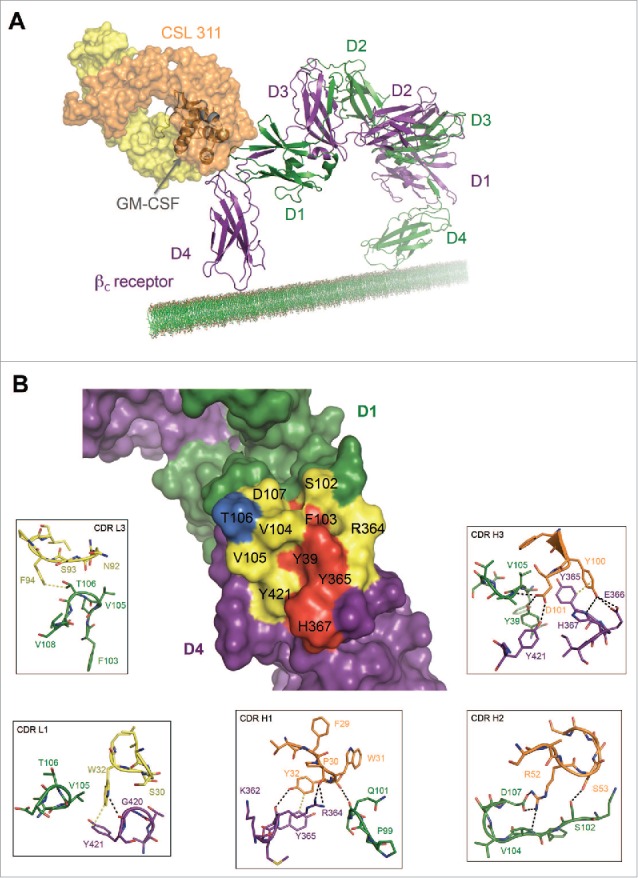
The Site 2 Interaction interface between CSL311 and the βc receptor. (A) Superimposition of the βc/CSL311 Fab complex on the GM-CSF receptor ternary structure, showing the overlap of the interaction interface between CSL311 Fab (heavy chain shown in orange and light chain in yellow) and GM-CSF (gray). The βc dimer from the GM-CSF ternary structure is colored in green and purple. (B) A surface representation of the key residues on the βc dimer that interact with CSL311 is shown. Individual residues are colored to indicate the effect of specific alanine substitution mutations on CSL311 binding affinity: mutations that lead to no binding or negligible binding are colored in red, mutations that reduce binding to the 10−5 to 10−7 M range are shown in yellow and mutations that improve binding are shown in blue. The detailed interactions involving CDRs H1-H3 and CDR L1 and L3 are shown in the adjoining zoom-in panels. Polar interactions are shown as black broken lines and key van der Waals interactions are shown in yellow. All figures were made using PyMOL.
