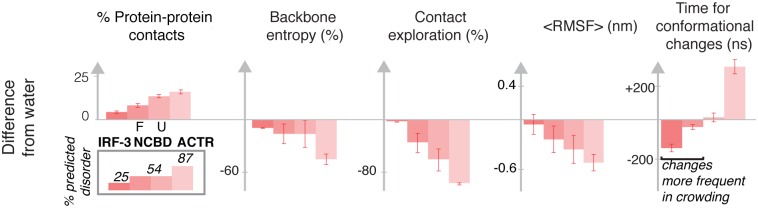
Fig 6. Effects of protein crowding depend on the protein disorder.
From the left: the percentage of protein-protein contacts (from the total contacts that a protein forms) is proportional to the intrinsic disorder of each protein (calculated with PONDR-FIT) [39]; the same trend is followed by the other observables that report the decrease in the conformational exploration compared to the simulation in water (calculated globally as the backbone conformational entropy and locally as the % of explored intra-protein contacts;) and the change in protein dynamics (calculated locally as the average local root mean square fluctuation RMSF (nm) and globally as the time between conformational changes). Values are averaged from the crowder concentrations. This trend was not observed in PEG500, see S11 Fig for a comparison.