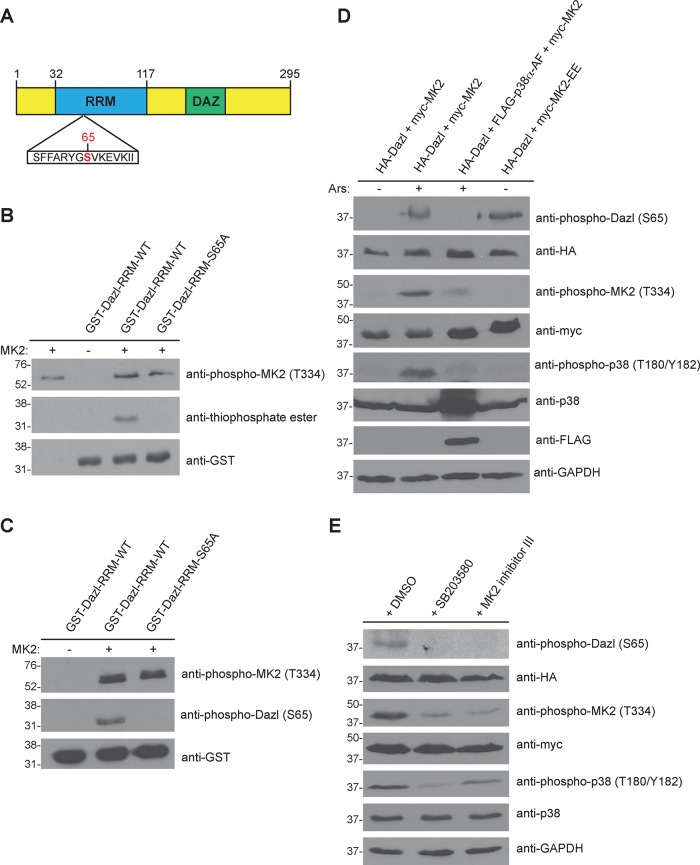
FIGURE 2:
MK2 phosphorylates Dazl at S65. (A) Domain architecture of human Dazl. DAZ, deleted in azoospermia domain; RRM, RNA recognition motif. The putative MK2 phosphorylation site (S65) is indicated in red. (B) MK2-mediated phosphorylation of Dazl-S65 in vitro. Wild-type or S65A GST-Dazl-RRM (residues 32–117) fusion proteins were incubated with active recombinant MK2 and ATPγS, followed by incubation with PNBM. Kinase reactions were resolved by SDS–PAGE (on two separate gels), followed by immunoblotting with the indicated antibodies. (C) Detection of Dazl-S65 phosphorylation using a phosphospecific antibody. GST-Dazl RRM (wild-type or S65A) fusion proteins were incubated with active recombinant MK2 and ATP. Kinase reactions were resolved by SDS–PAGE (on two separate gels), followed by immunoblotting with the indicated antibodies. (D) Alteration of Dazl-S65 phosphorylation status via genetic manipulation of the p38-MK2 signaling pathway. HEK293 cells were transfected with the indicated constructs and either stimulated or not with sodium arsenite (Ars) and lysed. Cell lysates were split, resolved by SDS–PAGE (on eight separate gels), and immunoblotted with the indicated antibodies. Western blots shown are representative of three independent experiments. (E) Reduced Dazl-S65 phosphorylation by chemical inhibition of the p38-MK2 signaling pathway. HEK293 cells were transfected with the indicated constructs and pretreated for 30 min with DMSO (vehicle) only, the p38 inhibitor SB20350 (10 μM), or MK2 inhibitor III (20 μM). Cells were then stimulated with sodium arsenite and lysed. Cell lysates were split, resolved by SDS–PAGE (on seven separate gels), and immunoblotted with the indicated antibodies. Western blots shown are representative of three independent experiments.