Fig. 3.
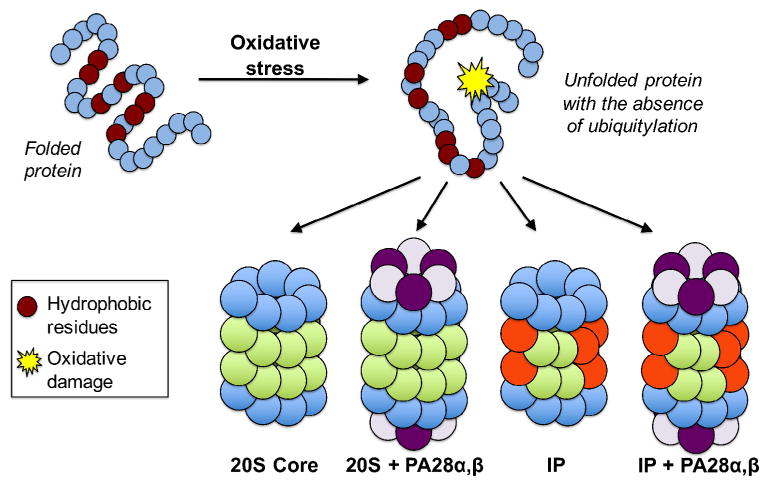
Mechanism of action for the degradation of oxidized proteins. Oxidative stress results in damage to proteins that cause them to unfold, thus revealing hydrophobic residues that are prone to aggregation. The 20S proteasome recognizes these hydrophobic patches on the surface of a substrate protein, which can promote the opening of the outer α-ring. In addition, both the immunoproteasome and the 20S proteasome bound to Pa28 regulators have a preference for degrading oxidatively damaged proteins.