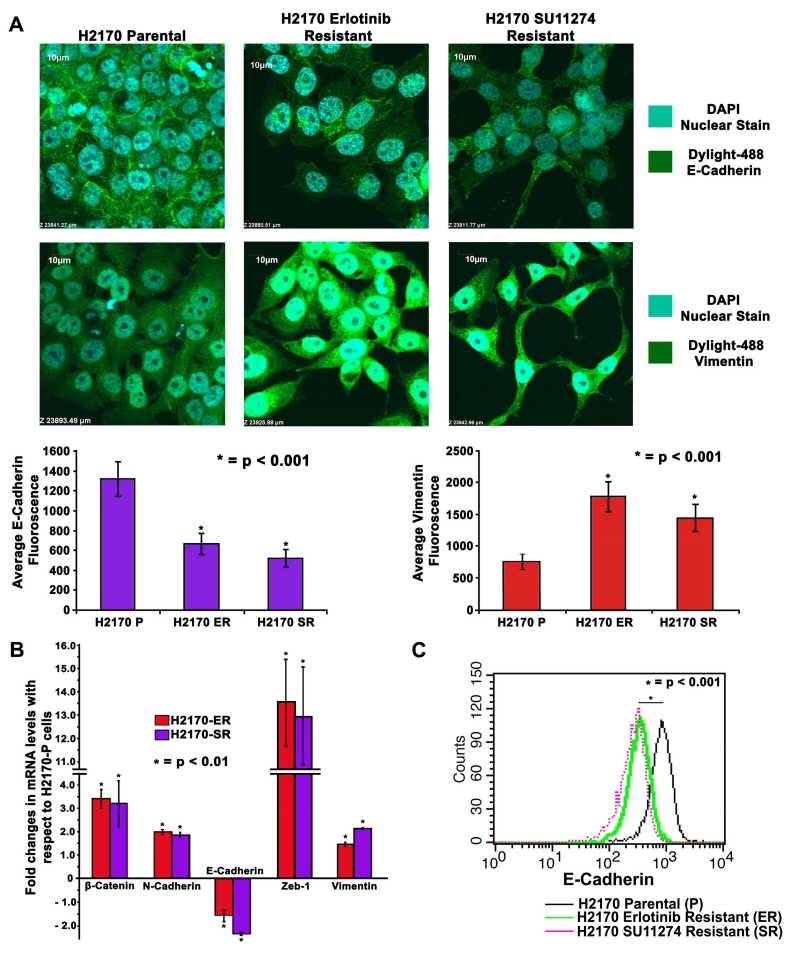
Fig 2.
Changes in cell morphology and modulations in expression of EMT related proteins in TKI-resistant NSCLC cells (A) 2×104 cells per well in an 8-well chamber slide were plated, fixed and probed with Vimentin and E-Cadherin antibodies. DAPI was used as a nuclear stain while Dylight-488 conjugated secondary antibodies were used for detection of Vimentin and E-Cadherin. Images were captured using an Olympus fv10i confocal microscope and fluorescence quantification was performed using the Olympus Fluoview image analysis software. Vimentin filaments were found to be upregulated and E-Cadherin was observed to be downregulated in H2170-ER and H2170-SR cells when compared to H2170-P cells. (B) 2.5×105 cells were seeded in 35mm dishes and allowed to grow for 24 hr, and kept in serum free medium for 24 hr. Total RNA was collected and quantified. Subsequently, 1μg of total RNA was used to obtain cDNA to determine expression of genes of interest using qPCR. β-Catenin, N-Cadherin, Zeb-1 and Vimentin were found upregulated while E-Cadherin was found downregulated in H2170 TKI-resistant cells when compared to parental cells. The data was normalized with GAPDH and graphically represented relative to expression of respective genes in H2170-P cells. (C) H2170-P, ER and SR cells were collected using Accutase cell detachment media and then probed with E-Cadherin antibody conjugated with Alexa Fluor 647. The pellet was then washed and re-suspended in Flow cytometry buffer (PBS + 0.5% BSA + 5mM EDTA). Equal numbers of cells were analyzed for all the cell types and histogram for fluorescence intensity was plotted. The results show that there is a loss in E-Cadherin signal in H2170-ER and H2170-SR cells when compared to H2170-P cells.