Figure 8.
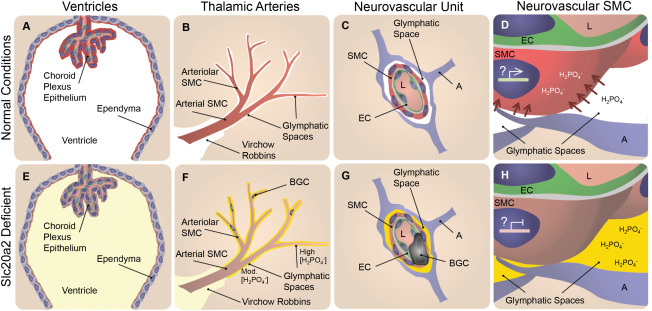
Slc20a2 deficiency‐associated high CSF phosphate and SMC susceptibility to calcification may result in BGC in a 2‐hit mechanism. In normal conditions phosphate CSF concentrations are maintained at homeostatic levels as indicated in white in A‐D. SLC20A2 is poised to regulate CSF phosphate and is localized to the choroid plexus and ependyma (red in A), and the neurovascular SMCs (red in B‐D) adjacent to CSF‐containing glymphatic spaces. In Slc20a2 deficient conditions, these tissues contain reduced levels of SLC20A2 indicated in brown (E‐H). Slc20a2 deficiency leads to higher CSF phosphate concentrations in glymphatic spaces as indicated in yellow (E‐H). The glymphatic spaces are delineated by astrocytes and permeated by astrocyte endfeet, creating pockets. Fluid flow through this porous and segmented structure (G and H), is assumed to increase retention parameters caused by turbulent flow and increased surface‐ion interactions mediated by Brownian Forces. In this case a higher concentration of phosphate likely accumulates in glymphatic spaces that surround the exterior of smaller vessels, indicated by the yellow gradient and segmentation by astrocyte endfeet (F‐H). In normal conditions, SLC20A2 may act to promote SMC phosphate clearance from arteriolar glymphatic spaces (red arrows in D), and SLC20A2 plays a currently unidentified protective role against SMC calcification, illustrated by active gene expression in a SMC nucleus (D). In disease conditions, SLC20A2 deficiency results in high CSF phosphate concentrations compounded by the loss of protective roles against SMC calcification (H). We pose the hypothesis that these abnormalities lead to BGC in a 2‐hit mechanism. Abbreviations: BGC=basal ganglia calcification; SMC = smooth muscle cell; EC = endothelial cell; A = astrocyte; L = lumen.
