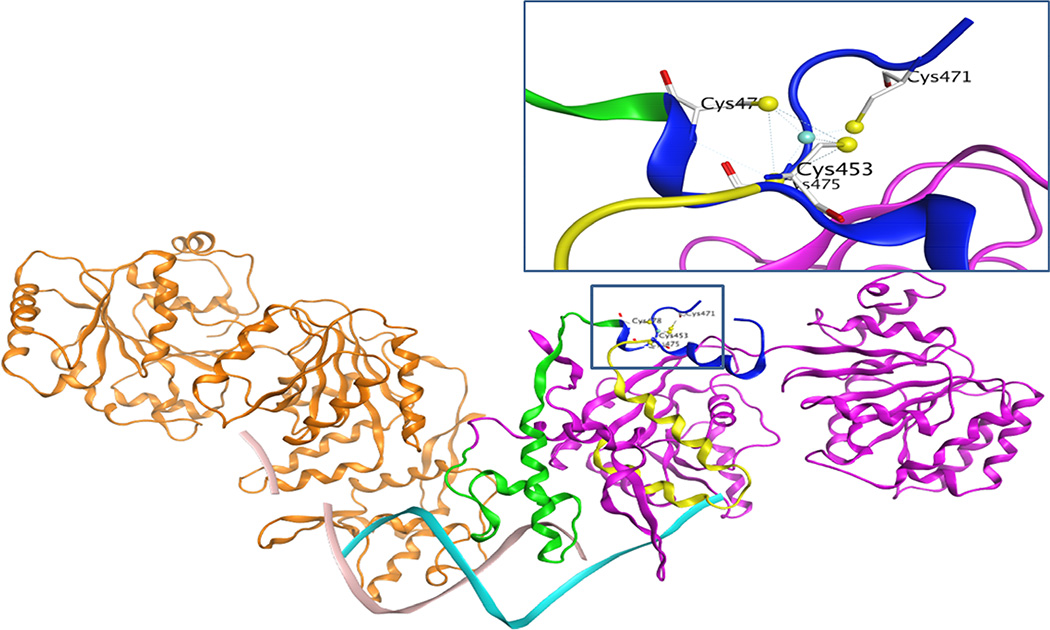
Figure 9. Differential contribution of conserved cysteine residues in RECQ1 structure and function.
Homology modeling shows that the four cysteine residues located on the loop of a side chain could act like a hinge to assist RECQ1 interaction with the DNA. Cysteine residues 453, 475, and 478 are located on the part of RECQ1 protein shown as the ribbon highlighted green and yellow. These parts of the protein are directly going down to interact with DNA, indicating a major contribution for a specific protein conformation and DNA binding activity. On the other hand, the cysteine 471 is located on the far end of the protein in a flexible loop, and one end of the loop is separated by a strong interaction of cysteine 453 with zinc. The newly solved crystal structure of the truncated RECQ1 protein (PDB ID: 4U7D) did not show data for this loop, perhaps due to its intrinsically unstructured character.