Fig. 4.
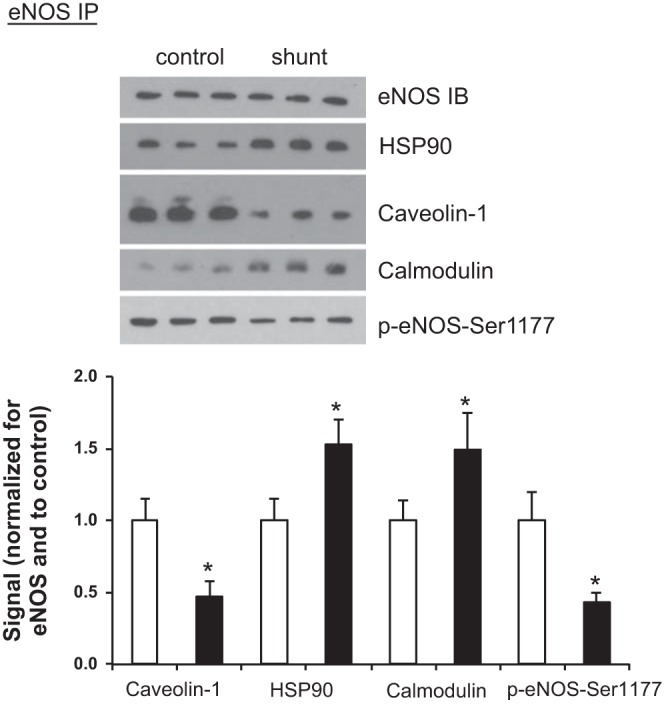
eNOS protein-protein interactions and Ser1177 phosphorylation in LECs. Following eNOS immunoprecipitation (IP) in control and shunt LECs, Western blots were performed for eNOS [immunoblot (IB)], 90-kDa heat shock protein (HSP90), caveolin-1, calmodulin, and phospho-eNOS-serine-1177. Normalized to control LECs, the steric inhibitor caveolin-1 associated with eNOS is decreased in shunt LECs (0.47 ± 0.11); conversely, the relative amount of the chaperone HSP90 associated with eNOS increases in shunt LECs (1.53 ± 0.17), and the relative amount of calmodulin associated with eNOS increases in shunt LECs to 1.49 ± 0.25. The relative amount of phosphorylated Ser1177 decreases in shunt LECs (0.43 ± 0.07). Data are shown as mean ratios [caveolin-1, HSP90, calmodulin, or phospho (p)-eNOS-Ser1177/eNOS] ± SD and normalized to control. For all experiments, n = 3 control, 3 shunt, with *P < 0.05.
