Fig. 3.
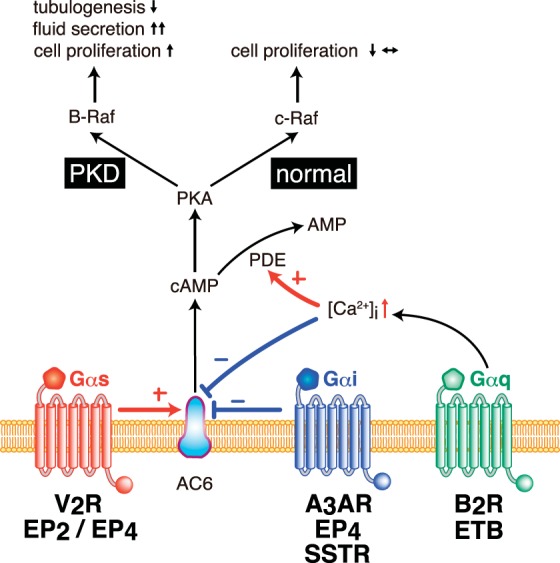
Overview of cAMP regulation by distinct heterotrimeric G protein subunits in renal tubular epithelial cells. Intracellular levels of cAMP have been shown to be regulated by an integration of numerous GPCR pathways in renal tubular epithelial cells. Adenylyl cyclase type 6 (AC6) could be either directly activated by GPCR/Gαs or inhibited by GPCR/Gαi. In addition, GPCR/Gαq signaling could stimulate intracellular calcium, which could be involved in reducing AC6 activity or accelerating cAMP catalysis by phosphodiesterase (PDE) activation. Upon changes in cAMP levels, protein kinase A (PKA)-dependent phosphorylation of Raf kinase has been shown to be involved in controlling distinct cellular functions, particularly cell proliferation. In normal renal tubular epithelial cells, c-Raf (Raf-1) kinase activates MEK/ERK1/2 signaling to prevent or reduce the activation of cell proliferative pathways. In cystic PKD epithelial cells, PKA has been shown to activate B-Raf kinase to promote increased cell proliferation, increased fluid secretion, and reduced tubulogenesis. V2R, vasopressin V2 receptor; EP2/EP4, E-prostanoid 2 or 4 receptor; A3AR, adenosine A3 receptor; SSTR, somatostatin receptor; B2R, bradykinin type 2 receptor; ETB, endothelin type B receptor; +, activate; −, inhibit.
