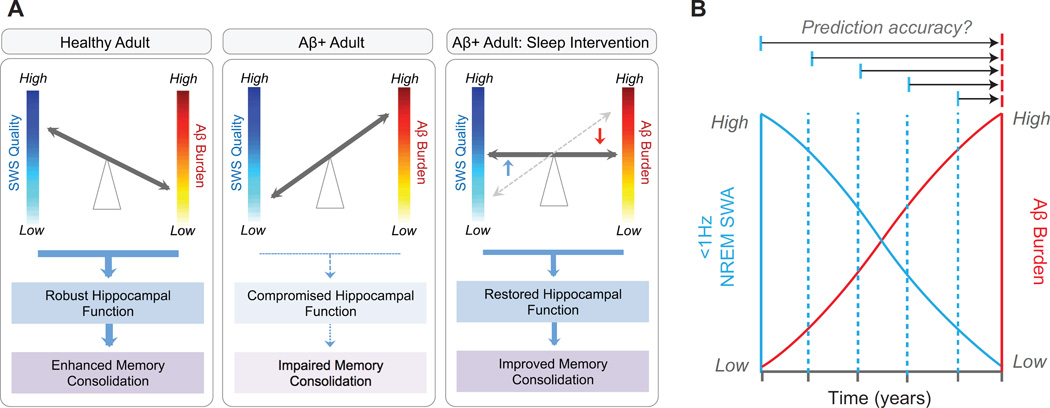
Figure 3.
Proposed consequences of the reciprocal relationship between Aβ, <1Hz NREM SWA and memory functioning under different circumstances, and the potential utility of sleep as a novel biomarker. In healthy older adults with low Aβ burden (a, left panel), NREM SWS quality is high, thereby facilitating hippocampus-dependent memory consolidation. In Aβ+ older adults (a, middle panel), NREM SWS quality is low, resulting in compromised memory consolidation. However, should NREM SWS quality be rescued through therapeutic sleep intervention in Aβ+ older adults (a, right panel), memory consolidation should be improved through two non-mutually exclusive pathways: i) by minimizing the negative impact of Aβ burden on sleep-dependent memory processing, and/or ii) through facilitating greater glymphatic Aβ clearance. (b) Since <1Hz NREM SWA is associated with Aβ burden in healthy older adults before MCI or AD onset, it is possible that this measure may offer diagnostic utility as a noninvasive biomarker of Aβ burden and AD risk. Longitudinal studies have the ability to examine the diagnostic potential of <1Hz NREM SWA, not only as a static, surrogate marker of current Aβ burden, but as a predictive biomarker that forecasts Aβ accumulation or risk of conversion to AD years in advance (multiple horizontal arrow tests). Abbreviations: Aβ, amyloid-β protein; Aβ + adult, an older adult with Aβ pathology; SWS, slow wave sleep; and NREM SWA, non-rapid-eye-movement slow wave activity.