Abstract
Cesarean scar pregnancy (CSP) is a rare, but life-threatening type of ectopic pregnancy. An exact and early diagnosis of CSP is very important for prognosis. The aim of the present study was to describe 4 women with CSP and discuss their clinical presentations, diagnoses, and various management options along with the published literature. Four women with a suspicion of CSP or cervical pregnancy were referred to our hospital between August 2013 and January 2014. All the patients were counseled about medical management options. After the treatment, serum beta human chorionic gonadotropin (ß-hCG) levels were followed weekly until they reached <5 mIU/mL. All the patients were diagnosed at the first trimester, with the average gestational age of 6.4±0.9 weeks. Treatment was systemic methotrexate (MTX) treatment in 3 of the 4 women, requiring no further intervention. The remaining 1 woman was treated with an intragestational administration of MTX. The mean time passed until ß-hCG reached <5 mIU/mL was 10.2±2.9 (range, 8–14) weeks, and the mean time passed until the gestational sac resolved was 21.5±3.5 (range, 18–25) weeks. Based on this limited number of case-series experience, it seems that CSP should be treated conservatively even if there are visible fetal cardiac activity, fetal poles, large gestational sacs, and high initial ß-hCG levels. Although the complete remission of the lesion takes a relatively long time, medical management via a noninvasive approach and follow-up should be tried as the first choice of therapy.
Keywords: Cesarean scar pregnancy, Ectopic pregnancy, Methotrexate, Treatment
What’s Known
Current management options for cesarean scar pregnancy (CSP) are based on case reports and small case series; therefore, an ideal treatment modality is still under debate.
What’s New
Our results, albeit in a small study population, lend further support for the conservative management of CSP even if there are visible fetal cardiac activity, fetal poles, large gestational sacs, and high initial ß-hCG levels.
Local administration of medical therapy may shorten the duration of complete remission.
Introduction
Cesarean scar pregnancy (CSP) is a rare, albeit life-threatening, type of ectopic pregnancy in which the embryo implants in an iatrogenic Cesarean scar left in the uterus. CSP is a distinct entity compared with other types of ectopic pregnancies because it never occurs in the first pregnancy and it never occurs in a woman with no previous Cesarean delivery. The reported rate of CSP is 1 in 2,000 and accounts for 6% of ectopic pregnancies among women with a prior Cesarean delivery.1 An exact and early diagnosis of CSP is very important for prognosis because it can result in life-threatening conditions and even maternal death.2,3 Here, we describe 4 women with CSP and discuss their clinical presentations, diagnoses, and various management options, especially medical management of CSP, along with the published literature.
Case Presentation
Four women with a suspicion of CSP or cervical pregnancy were referred to our hospital between August 2013 and January 2014. All the patients had detailed ultrasonographic (USG) assessment and measurement of serial serum beta human chorionic gonadotropin (ß-hCG) levels. All the patients were counseled about medical management. The benefits and potential risks of methotrexate (MTX) treatment were explained, and written informed consents were obtained. Before the treatment, liver and kidney functions were evaluated. The age, parity, USG assessment, serum ß-hCG level, management, and follow-up period of the 4 patients are summarized in table 1. None of the women had any clinical symptoms such as vaginal bleeding and lower abdominal pain. The size of the gestational sac and the ß-hCG levels were followed until the gestational sac shrank and the ß-hCG level reached <5 mIU/mL (defined as undetectable level).
Table 1.
Summary of the characteristics of the 4 patients treated with different conservative treatment methods
| Patient # | Age (years) | Parity/n. of CSs | USG of scar gestation | EGA (weeks)* | FKA* | InitialßhCG (mIU/mL)* | Systemic therapy | Local injection | Time passed until ßhCG reached <5 mIU/ml (weeks) | Time passed until GS resolved (weeks) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 32 | 1/1 | CRL: 10.8 mm | 7.3 | Yes | 124.106 | 1 dose of MTX, followed by 4 doses of MTX and LCV | 14 | 25 | |
| 2 | 37 | 3/2 | CRL: 5.8 mm | 6.3 | Yes | 16.654 | 1 dose of MTX, followed by 4 doses of MTX and LCV | 11 | 24 | |
| 3 | 28 | 1/1 | GS: 7×6×6.8 mm, with yolk sac | 5.3 | No | 5.280 | 2 doses of MTX | 8 | 18 | |
| 4 | 35 | 2/1 | CRL: 8.8 mm | 6.6 | Yes | 52.248 | MTX and KCl | 8 | 19 |
At the time when the procedure was performed. USG: Ultrasonography; CS: Cesarean section; CRL: Crown-rump length; EGA: Estimated gestational age; FKA: Fetal cardiac activity; β-hCG: Beta human chorionic gonadotropin; GS: Gestational sac; MTX: Methotrexate; LCV: Leucovorin; KCl: Potassium chloride
The USG assessment of the 4 patients revealed common findings: a well defined gestational sac with or without a crown-rump length, a closed internal cervical ostium, a normal cervical length, and an empty uterus (figure 1).
Figure 1.
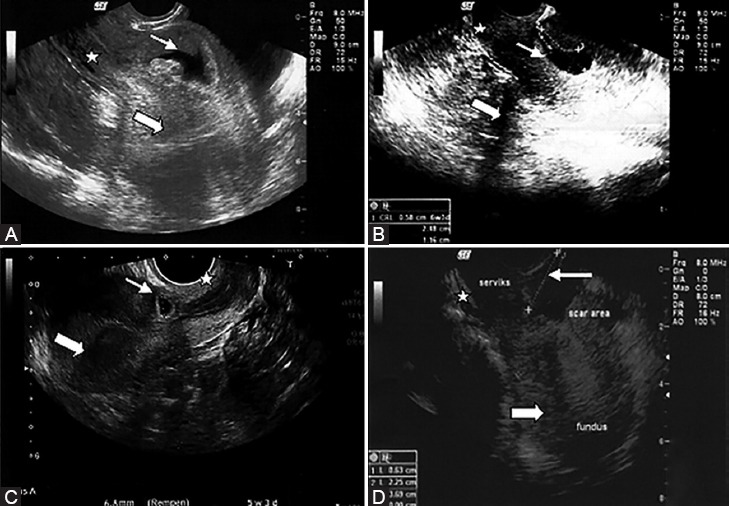
Transvaginal ultrasonography of the Cesarean scar pregnancy of the 4 women in the study is depicted here. The midline sagittal images demonstrate gestational sacs (small arrows) implanted at the isthmic region between a closed cervix (star) and an empty uterine cavity (large arrows) and the anatomical location of previous Cesarean scars (A-D). A crown-rump length is distinguished inside the gestational sac (A,B).
In all the pregnancies, the intramural location of the gestational sac was consistent with the previous Cesarean scar. Moreover, 3 of the women had a fetal cardiac activity. Since these clinical (high serum ß-hCG levels) and USG findings were compatible with CSP, further evaluations were considered unnecessary.
Systemic MTX treatment with a single-dose weight-based protocol (1 mg/kg intramuscular) was given. Serum ß-hCG levels were checked before treatment and then on the 4th and 7th days of treatment. In the first and second women, the ß-hCG levels increased from day 4 to day 7 from 128.318 to 132.493 mIU/mL and from 30.213 to 34.600 mIU/mL, respectively (figure 2).
Figure 2.
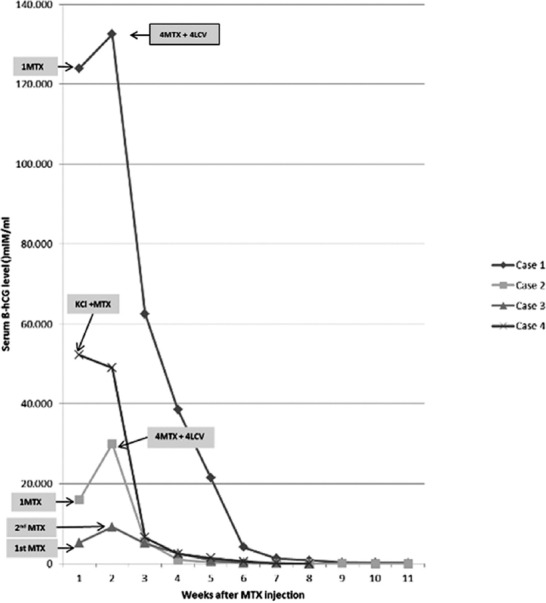
Changes in serum beta human chorionic gonadotropin (ß-hCG) levels of the 4 patients with Cesarean scar pregnancy before and after the medical treatment are illustrated here. The values on the curve represent the ß-hCG measurements at each given week. MTX: Methotrexate; LCV: Leucovorin; KCl: Potassium chloride.
Since the expected serum ß-hCG level decrease was not achieved, multi-dose MTX and leucovorin were administered consecutively (in 8 days: 4 days of MTX [1 mg/d] and 4 alternative days of leucovorin [0.1 mg/d]). After the last dose, the first and second patients’ ß-hCG levels were 62.514 mIU/mL and 5.542 mIU/mL, respectively. The ß-hCG levels reached <5 mIU/mL 14 and 11 weeks after the first day of medical treatment. The complete remission of the gestational sac diameter took approximately 6 months. In the third patient, a single-dose systemic MTX (1 mg/kg) was administered, and the serum ß-hCG level increased to 9105 mIU/mL on day 4 and remained elevated at 9225 mIU/mL on day 7 (figure 2). A repeated USG scan revealed a stable intramural hypoechogenic gestational sac in the lower uterine segment. A second single-dose MTX was administered for persistency. Thereafter, the ß-hCG level decreased to 5026 mIU/mL and 2770 mIU/mL on days 4 and 7, respectively. Eight weeks after the initial MTX dose, the ß-hCG level was undetectable and USG revealed a stable 2.5×3.8×2.7 cm heterogeneous mass in the lower uterine segment. The mass eventually resolved 18 weeks after the treatment. In the fourth patient, after extensive counseling, the pregnancy was terminated through a transvaginal USG-guided injection of potassium chloride (0.1 nmol) into the fetal cardiac cavity with the resultant cessation of the cardiac activity, followed by the administration of 50 mg of MTX into the gestational sac. The serum ß-hCG level decreased to 49.003 mIU/mL on day 4 and 8.640 mIU/mL on day 7. Eight weeks after the injection, the ß-hCG level reached the undetectable level. A follow-up USG showed the resolution of the gestational sac after 19 weeks.
None of the patients experienced any adverse effects related to the treatment or reported any unexpected vaginal bleeding during the follow-up. Their regular menstrual cycles started within 1 month after their ß-hCG levels decreased to <5 mIU/mL.
Discussion
The rate of CSP is expected to rise in the future due to the increasing number of Cesarean sections. However, the ideal treatment protocol is still unknown. The reasons for the ectopic implantation of the conceptus into the Cesarean scar as well as its diagnosis and treatment need further evaluation. Indeed, the exact cause of CSP is ambiguous. Jurkovic et al.4 hypothesized that a microscopic defect or the microtubular tract in the Cesarean scar might be related to CSP by a leading invasion of the conceptus into the myometrium. More than half of all cases of CSP were in women with 1 prior Cesarean delivery.1 Similarly, 3 of our 4 patients had 1 Cesarean delivery.
The clinical manifestations of CSP range from no symptoms to acute-onset lower abdominal pain, vaginal bleeding, and even hypovolemic shock.2,5 In a review of 57 patients with CSP, 36.8% of the women were asymptomatic, 38.6% presented with painless vaginal bleeding, and only a minority (24.6%) presented with abdominal pain.1 All of our patients were asymptomatic and referred to our hospital as a result of suspicious USG examinations.
CSP diagnosis is confirmed by USG assessment.5,6 The sensitivity of transvaginal ultrasound is 86.4%.1 Magnetic resonance imaging and hysteroscopy may be used for further evaluation of pregnancy location, but these are not compulsory for the diagnosis.7 In our patients, no additional diagnostic assessment other than USG was required.
Several treatment options for CSP such as expectant, medical, and surgical management have been proposed. Nonetheless, the most appropriate one has yet to be clearly determined. The expectation in expectant management is that the conceptional material will be reabsorbed and/or expelled by itself against the risk of uterine rupture and hemorrhage.7 Medical management is systemic and is done via a local administration of MTX, potassium chloride, or a combination of these agents by laparoscopic or USG-guided injections.8,9 Several surgical options have been suggested such as uterine artery embolization, hysteroscopy,10 and D&C.11 Myometrial wedge excision and hysterectomy may also be needed when there are life-threatening clinical presentations.12
Available data on expectant management show that it is rarely successful.7,12 Rotas et al.1 showed that uterine rupture with severe hemorrhage occurred in half of the women who were only followed up without treatment. In a hemodynamically stable patient, medical or surgical options may be considered with the aim of eliminating the gestational sac and retaining fertility.7 The administration of local or systemic MTX has shown high success rates. A single intramuscular dose of 50 mg/m2 of MTX can be used for CSP with a gestational age of less than 6–8 weeks without fetal cardiac activity.13 In some reviews, it is mentioned that systemic treatment with MTX is successful if the patient’s ß-hCG level is <5000 mIU/mL; nevertheless, additional or alternative treatment interventions are necessary when the ß-hCG level is >6000 mIU/mL.1,12 In our first 3 patients, since a single-dose MTX treatment was not adequate, complete remission was obtained with complementary medical treatment.
It can be debated whether systemic MTX is the treatment of choice. Due to the impaired vascularization of this fibrous tissue, the penetration of MTX into the fetal sac may be insufficient. For this reason, some investigators have advocated a local injection of MTX into the gestational sac.9,12 Jurkovic et al.4 reported 18 cases of CSP. Four of these cases were successfully treated with local injections of MTX and potassium chloride. In our fourth patient, after the local injection of potassium chloride and MTX, complete remission was obtained within 8 weeks.
In 3 of our 4 patients, the serum ß-hCG levels initially increased after the first systemic MTX treatment but after the complementary medical treatment, they showed a steady decline. In contrast, local injections of potassium chloride and MTX caused an immediate decline in the serum ß-hCG levels (figure 2).
The serum ß-hCG level is a good marker for following the response of patients to MTX treatment. Although there are no guidelines on the optimal follow-up, it is recommended to measure the ß-hCG level weekly until it reaches <5 mIU/mL.14 Another follow-up method is serial USG examinations. In our patients, the decrease in the ß-hCG levels and the shrinkage of the CSP mass were found to be inconsistent. Furthermore, the ß-hCG levels reached the undetectable level within 8–14 weeks and the remission of the conceptions took a minimum of 18 weeks.
Conclusion
Based on this limited number of cases, it seems that CSP should be treated conservatively even if there are visible fetal cardiac activity, fetal poles, large gestational sacs, and high initial ß-hCG levels. Although the complete remission of the lesion takes a relatively long time, medical management via a noninvasive approach and follow-up should be tried as the first choice of therapy.
Conflict of Interest: None declared.
References
- 1.Rotas MA, Haberman S, Levgur M. Cesarean scar ectopic pregnancies: etiology, diagnosis, and management. Obstet Gynecol. 2006;107:1373–81. doi: 10.1097/01.AOG.0000218690.24494.ce. [DOI] [PubMed] [Google Scholar]
- 2.Einenkel J, Stumpp P, Kosling S, Horn LC, Hockel M. A misdiagnosed case of caesarean scar pregnancy. Arch Gynecol Obstet. 2005;271:178–81. doi: 10.1007/s00404-004-0683-1. [DOI] [PubMed] [Google Scholar]
- 3.Weimin W, Wenqing L. Effect of early pregnancy on a previous lower segment cesarean section scar. Int J Gynaecol Obstet. 2002;77:201–7. doi: 10.1016/S0020-7292(02)00018-8. [DOI] [PubMed] [Google Scholar]
- 4.Jurkovic D, Hillaby K, Woelfer B, Lawrence A, Salim R, Elson CJ. First-trimester diagnosis and management of pregnancies implanted into the lower uterine segment Cesarean section scar. Ultrasound Obstet Gynecol. 2003;21:220–7. doi: 10.1002/uog.56. [DOI] [PubMed] [Google Scholar]
- 5.Vial Y, Petignat P, Hohlfeld P. Pregnancy in a cesarean scar. Ultrasound Obstet Gynecol. 2000;16:592–3. doi: 10.1046/j.1469-0705.2000.00300-2.x. [DOI] [PubMed] [Google Scholar]
- 6.McKenna DA, Poder L, Goldman M, Goldstein RB. Role of sonography in the recognition, assessment, and treatment of cesarean scar ectopic pregnancies. J Ultrasound Med. 2008;27:779–83. doi: 10.7863/jum.2008.27.5.779. [DOI] [PubMed] [Google Scholar]
- 7.Maymon R, Halperin R, Mendlovic S, Schneider D, Herman A. Ectopic pregnancies in a Caesarean scar: review of the medical approach to an iatrogenic complication. Hum Reprod Update. 2004;10:515–23. doi: 10.1093/humupd/dmh042. [DOI] [PubMed] [Google Scholar]
- 8.Holland MG, Bienstock JL. Recurrent ectopic pregnancy in a cesarean scar. Obstet Gynecol. 2008;111:541–5. doi: 10.1097/01.AOG.0000287295.39149.bd. [DOI] [PubMed] [Google Scholar]
- 9.Hartung J, Meckies J. Management of a case of uterine scar pregnancy by transabdominal potassium chloride injection. Ultrasound Obstet Gynecol. 2003;21:94–5. doi: 10.1002/uog.12. [DOI] [PubMed] [Google Scholar]
- 10.Hois EL, Hibbeln JF, Alonzo MJ, Chen ME, Freimanis MG. Ectopic pregnancy in a cesarean section scar treated with intramuscular methotrexate and bilateral uterine artery embolization. J Clin Ultrasound. 2008;36:123–7. doi: 10.1002/jcu.20374. [DOI] [PubMed] [Google Scholar]
- 11.Wang CJ, Yuen LT, Chao AS, Lee CL, Yen CF, Soong YK. Caesarean scar pregnancy successfully treated by operative hysteroscopy and suction curettage. BJOG. 2005;112:839–40. doi: 10.1111/j.1471-0528.2005.00532.x. [DOI] [PubMed] [Google Scholar]
- 12.Seow KM, Huang LW, Lin YH, Lin MY, Tsai YL, Hwang JL. Cesarean scar pregnancy: issues in management. Ultrasound Obstet Gynecol. 2004;23:247–53. doi: 10.1002/uog.974. [DOI] [PubMed] [Google Scholar]
- 13.Haimov-Kochman R, Sciaky-Tamir Y, Yanai N, Yagel S. Conservative management of two ectopic pregnancies implanted in previous uterine scars. Ultrasound Obstet Gynecol. 2002;19:616–9. doi: 10.1046/j.1469-0705.2002.00719.x. [DOI] [PubMed] [Google Scholar]
- 14.Ash A, Smith A, Maxwell D. Caesarean scar pregnancy. BJOG. 2007;114:253–63. doi: 10.1111/j.1471-0528.2006.01237.x. [DOI] [PubMed] [Google Scholar]


