Abstract
Plasmablastic lymphoma is a rare and aggressive diffuse large B-cell lymphoma commonly associated with Epstein-Barr virus co-infection that most often occurs in the context of human immunodeficiency virus infection. Therefore, its immune escape strategy may involve the upregulation of immune-checkpoint proteins allowing the tumor immune evasion. However, the expression of these molecules was poorly studied in this lymphoma. We have investigated 82 plasmablastic lymphoma cases of whom half were Epstein-Barr virus positive. Although they harbored similar pathological features, Epstein-Barr virus positive plasmablastic lymphomas showed a significant increase in MYC gene rearrangement and had a better 2-year event-free survival than Epstein-Barr virus negative cases (P=0.049). Immunostains for programmed cell death-1, programmed cell death-ligand 1, indole 2,3-dioxygenase and dendritic cell specific C-type lectin showed a high or moderate expression by the microenvironment cells in 60%–72% of cases, whereas CD163 was expressed in almost all cases. Tumor cells also expressed programmed cell death-1 and its ligand in 22.5% and 5% of cases, respectively. Both Epstein-Barr virus positive and negative plasmablastic lymphomas exhibited a high immune-checkpoint score showing that it involves several pathways of immune escape. However, Epstein-Barr virus positive lymphomas exhibited a higher expression of programmed cell death-1 and its ligand in both malignant cells and microenvironment as compared to Epstein-Barr virus negative cases. In conclusion, plasmablastic lymphoma expresses immune-checkpoint proteins through both malignant cells and the tumor microenvironment. The expression of programmed cell death-1 and its ligand constitutes a strong rationale for testing monoclonal antibodies in this often chemoresistant disease.
Introduction
Plasmablastic lymphoma (PL) is a distinct entity of diffuse large B-cell lymphoma first described in 1997.1 It commonly occurs in the context of human immunodeficiency virus (HIV) infection or in association with other contexts of immunodeficiency such as autoimmune diseases, organ transplantation and in the elderly;2–5 few studies have identified PL in patients without immunodeficiency.6,7 Neoplastic cells resemble immunoblasts or plasmablasts with a B-cell terminal differentiation phenotype and they mostly have a non-germinal center (GC)-B subtype profile.2–8 These cells constitutively express the plasma cell antigen CD138 (syndecan-1) and often harbor immunoglobulin (Ig) chain restriction with no or weak expression of B-cell mature markers such as CD20, CD79a and PAX5.2,9 PL cells frequently express Epstein Barr virus (EBV) genome with type I latency, especially in HIV patients. On the other hand, EBV negative (EBV−) PL still remains poorly characterized, due in part to its rarity as this limits the assessment of distinctive clinicopathological features. In a recent study, Loghavi et al.6 analyzed 61 cases with treatment information available in 42. Among them, 17 were EBV− and had a worse event-free survival as compared to EBV+PL patients.6 Despite recent advances in lymphoma therapeutic strategies, EBV+PL, and to an even greater extent EBV−PL, still represent an aggressive lymphoma with adverse prognosis; therefore, novel therapy strategies are urgently needed.
Cancer cells, including lymphoma cells, are able to escape surveillance from the immune system by co-opting physiological mechanisms such as the programmed cell death-1 (PD-1) receptor pathway. By expressing PD-L1 on the tumor cell surface and engaging PD-1-positive infiltrating lymphocytes, tumors utilizing the PD-1 pathway can, therefore, evade an immune response by providing critical inhibitory signals that down-regulate T-cell function in the context of antigen recognition.10–13 Besides the PD-1/PD-L1 axis, the recruitment of M2 monocytes characterized by the expression of CD163 antigen has been involved in the tumor immune escape mechanism, especially through the production of immunosuppressive molecules such as the dendritic cell (DC)-specific C-type lectin DC-SIGN and/or the indole 2,3-dioxygenase (IDO).10,14–19 Accordingly, a blockade of these immune checkpoints alone or in combination with chemotherapy could be envisaged as an attractive therapeutic strategy.20–24 To the best of our knowledge, immune checkpoint profile molecule distribution has not previously been evaluated in PL. Given that EBV infection of malignant lymphocytes could be associated with PD-L1 overexpression,21,22,25,26 we hypothesized that PL cells could express PD-1/PD-L1 proteins and could constitute a prime target for PD-1 blockade. We, therefore, investigated the PD-1/PD-L1 axis and immune checkpoint profiles in PL and correlated these features with clinical presentation, histological findings and EBV status.
Methods
Patients
We reviewed 82 cases of PL diagnosed between 2008 and 2014, collected from 42,145 samples registered in Lymphopath, a government-supported network of expert hematopathologists which conducts a systematic review of all newly diagnosed or suspected lymphomas in France.27,28 The diagnosis of PL was based on histological criteria and the expression of plasma cell differentiation markers as described by the WHO classification.2 Patients with a prior diagnosis of plasma cell myeloma or with multiple bone lesions or other laboratory criteria supporting the diagnosis of myeloma were excluded from the study. The following data were collected: age, sex, disease location at presentation, an associated context of immunodeficiency (HIV infection, transplantation, autoimmune disease, immunosuppressive drugs), EBV serology, Ann Arbor staging, treatment and outcome, current status and date of last follow up.
Histological and immunohistochemical analysis
All cases were centrally reviewed. Institutional ethical approval was obtained in compliance with the Declaration of Helsinki. Paraffin tissue sections were processed for routine histopathological examination. For immunohistochemical examination, 3 μm-thick sections were tested using a Ventana Benchmark XT (Ventana, Tucson, AZ, USA).12,28 The antibodies used are detailed in Online Supplementary Table S1. Each case was scored as positive or negative for CD10, BCL6, MUM1, MYC protein, GCET1 and FOXP1 according to the cut-off points previously defined.29–31 The immune checkpoint score was based on the percentage of PD-1+ and PD-L1+ tumor cells (ranged from 0= <5% to 2= ≥30%) or the percentage of PD-1+, PD-L1+, IDO+, DC-SIGN+ or CD163+ immune cells (ranged from 0= <10% to 2= ≥30%) combined to the value corresponding to the staining intensity (ranged from 0= negative or low staining at + to 2= intense staining at +++); these were calculated by two pathologists (CL and BF), as recently published32 (Online Supplementary Table S2). The percentage threshold for positive PD-L1 staining in malignant cells used here is comparable to that already published.25,33,34
In situ hybridization for Epstein–Barr virus and FISH study for MYC, BCL2 and BCL6 rearrangement
Epstein-Barr virus detection was performed by in situ hybridization using EBV-encoded RNA (EBER) probes (Ventana Medical Systems). Fluorescence in situ hybridization (FISH) studies were performed using break-apart FISH DNA probes for cMYC/8q24, BCL2/18q21, and BCL6/3q27 (probes Y5410, Y5407 and Y5408; Dako) and were analyzed using Pannoramic 250 Flash digital microscopes (3DHISTECH, Hungary).35
Statistical analysis
Comparison of clinicopathological, immunological, and genetic features between EBV− and EBV+ patients was carried out using χ2 test (or Fisher exact test when required).
Event-free survival was determined from time of diagnosis until time of death, progression or last follow up. Survival curves were constructed by the Kaplan-Meier method. Survival distributions were compared with the log rank test. For co-variates with less than 20% of missing values and with a P-value<0.05 in the log rank test, Cox proportional hazards model was performed. Using a backward stepwise removal method, only significant co-variates were kept in the final Cox model. Statistical significance was set at P=0.05. Analyses were performed using GraphPrism and STATA v.13 software.
Results
Clinical features of EBV+ and EBV− plasmablastic lymphoma patients
Clinical features of EBV+ and EBV− plasmablastic lymphoma patients are shown in Table 1. Mean age at diagnosis was 62 years (range 22–88 years) with a male:female (M:F) ratio of 62:20. Diagnosis was made on the basis of a biopsy of a lymph node (n=17) or from an extranodal site (n=65): oral cavity (n=26), digestive tract (n=16), bone (n=5), soft tissue (n=7), respiratory tract (n=3), a kidney graft (n=2), testis (n=2), pleura (n=1), breast (n=1), orbital cavity (n=1), ureter (n=1). Half of the patients had underlying immunosuppression: HIV infection (41% of cases), and immunosuppressive (IS) treatment for kidney transplantation (3% of cases) or for autoimmune disease (5% of cases). Forty-three percent of patients (n=25) had no recorded history of immune suppression. We excluded from these categories patients for whom HIV status was not available (n=13) and patients who were HIV-negative and for whom a possible context of IS treatment could not be assessed (n=10). Half of the patients had localized disease, i.e. Ann Arbor stage I (29%) or II (21%); the remaining patients had disseminated disease, i.e. stage III (7%) or IV (43%). Extra-nodal disease was particularly common; the oral cavity was the most frequently observed site (46% of EBV+PL patients). Conversely, only 18% of EBV−PL patients had oral cavity involvement (P=0.016); moreover, a higher proportion of males was observed in EBV+PL cases versus EBV−PL cases (M:F ratio=32:7 vs. 26:12, respectively). EBV+PL patients tended to be more often HIV+ than EBV−PL patients (53% vs. 29%, respectively; P=0.05). No significant difference was observed with regard to age, bone marrow involvement and Ann Arbor stage between EBV+ and EBV−PL patients.
Table 1.
Clinical data in 82 plasmablastic lymphoma patients.
Nine patients were not treated for PL. Treatment details were available for 44 patients. Forty-two of 53 patients (79%) were treated with a sequential polychemotherapy, in association with autologous hematopoietic stem cell transplantation in 2 cases. Two patients were treated with radiotherapy alone. Most patients were treated with CHOP (cyclophosphamide, adriamycin, vincristine and prednisone) or CHOP-like regimens (n=18), in association with rituximab in 7 cases (Table 1). According to Cheson 1999 criteria,36 complete response (CR) was observed in 54%, 22% of cases had a partial response (PR), and 24% had persistent disease or died early.
Histological, immunophenotypic, and FISH analysis in EBV+ and EBV− plasmablastic lymphoma patients
All biopsies were diffusely infiltrated by a proliferation of large monomorphic predominantly plasmablast-like cells, and sometimes immunoblasts with round to oval nuclei usually containing a single central prominent nucleolus and eccentrically located in an abundant basophilic cytoplasm. Mitotic figures and areas of necrosis were frequent. Mature plasma cells in the background were uncommon and were not intermingled with the large tumor cells. Tumor cells were strongly and uniformly positive for CD138/VS38 with Ig light chain restriction detected in nearly 90% of cases. Neoplastic cells were usually EMA positive, and were mostly negative or focally and weakly positive for the B-cell marker CD79a, CD20 and PAX5 (Table 2). Tumor cells strongly expressed MUM1 (96%) and 28% of cases expressed FOXP1; they were negative for GCET1 and showed weak positivity for BCL6 (20%), while only 10% of cases expressed CD10. In addition, one-third of cases were positive for CD30 (36%) and BCL2 (32%). All cases were negative for ALK and HHV8 ruling out the diagnosis of ALK+ large B-cell lymphoma and diffuse large B-cell lymphoma arising in multicentric Castleman disease.37 Only 10% of cases stained weakly for CD56. Cyclin D1 was negative in all 42 cases tested.
Table 2.
Immunophenotype and FISH rearrangement studies in Epstein-Barr virus (EBV)+ and EBV− plasmablastic lymphomas.
Most cases (73%) were positive with anti-MYC protein antibody. FISH with the MYC break-apart probe was positive in 28% of cases tested (10 of 36). One case (1 of 31) showed a BCL6 rearrangement. No case was found rearranged for BCL2 (0 of 32). Notably, all cases that showed MYC rearrangement also had a strong expression of MYC protein in more than 80% of tumor cells. Moreover, 50% of cases with MYC rearrangement had BCL2 protein expression.
Half of the cases tested (39 of 77) expressed EBER in more than 90% of tumor cells. The morphological analysis of EBV+PL and EBV−PL cases showed similar features and harbored a similar phenotype. However, 43% of EBV+ PL tested (9 of 21) displayed an MYC rearrangement versus 6% in EBV− PL (1 of 15) (P=0.017).
Immune checkpoint expression in EBV-positive and EBV-negative plasmablastic lymphoma patients
Plasmablastic lymphoma biopsies showed a distinct pattern of expression for PD-1, PD-L1, CD163, IDO and DC-SIGN. Indeed, their microenvironment comprised a variable proportion of PD-1, PD-L1, CD163, IDO and DC-SIGN immune cells (Figure 1A–F). Among the immune cells, PD-1 and PD-L1 were primarily expressed by lymphoid cells and macrophages, respectively. However, some PL cases contained tumor cells, which expressed PD-L1 and/or PD-1 (Figure 1G and H). Immune checkpoint (ICP) score of PD-1, PD-L1, CD163 IDO and DC-SIGN was visually inspected on both tumor cells and immune cells from 42 available PLs and were quantified in terms of percentage of stained cells and intensity of staining, as described in the Methods section (Online Supplementary Table S2). As shown in Table 3, PD-L1 staining in immune cells ranged from low PD-L1 score with negative or weak/focal expression of PD-L1 (40%; n=15 of 40) to moderate or high PD-L1 score (60%; n=25 of 40). PD-1 staining ranged from low PD-1 score with negative or weak/focal expression (40%; n=13 of 32) to moderate or high PD-1 score (60%; n=19 of 32). Most of the PL cases were associated with a moderate or high score of CD163+ histiocytic cell infiltrates (97%; n=34 of 35). Histiocytic/dendritic cells of most PL showed an expression of IDO and DC-SIGN molecules with moderate or high IDO and DC-SIGN scores in 69% (n= 24 of 35) and in 72% (n=26 of 36) of PL cases, respectively. In addition, only a few CD8+ T cells expressed cytotoxic markers such as Granzyme B (data not shown).
Figure 1.
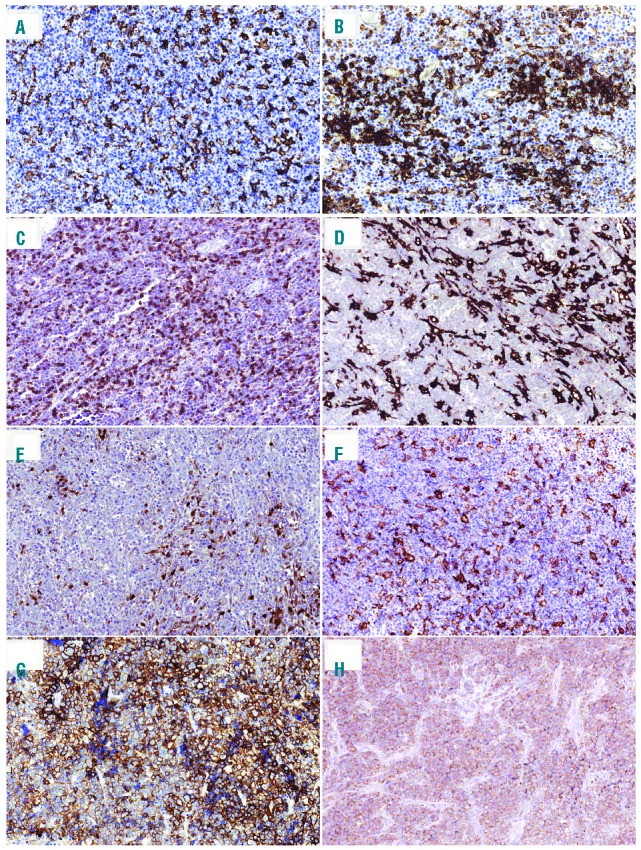
Immunohistochemical analysis of immune checkpoints in plasmablastic lymphomas (PL). (A and B) Representative cases of PL stained with anti-PD-L1 showing distinct membranous staining in intra-tumoral macrophages. (C) Representative case of PL stained with anti-PD-1 showing predominantly membranous staining in immune lymphoid cells. Example of (D) CD163, (E) IDO and (F) DC-SIGN stainings high-lighting macrophages in the PL microenvironment (×200). Representative cases of PL showing tumor cells that are positive for (G) PD-L1 or (H) for PD-1 (×200).
Table 3.
Immune checkpoint score in Epstein-Barr virus (EBV)+ and EBV− plasmablastic lymphoma immune cells.
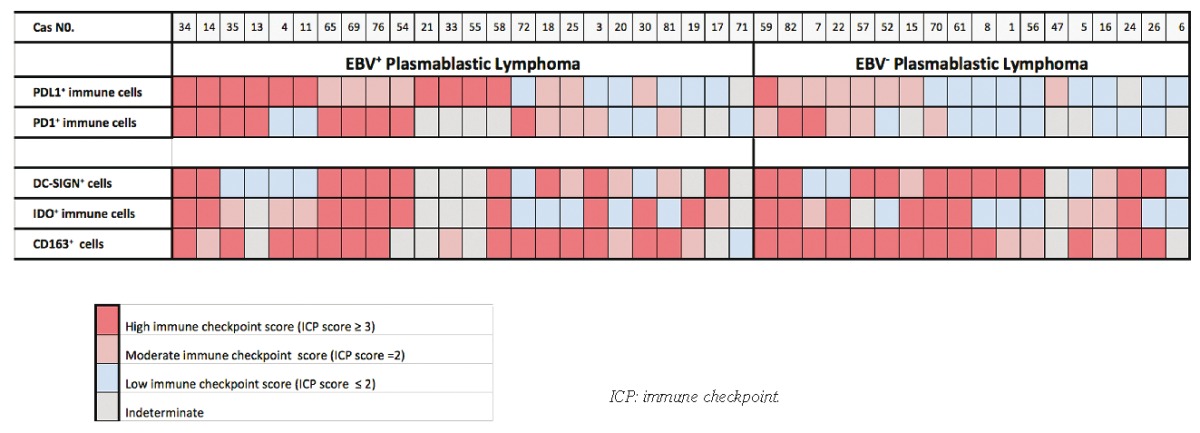
Interestingly, PD-1 was also expressed by tumor cells in 5% of PL cases (n=2 of 40), which exhibited a high PD-1 score, whereas PD-L1 was positive in tumor cells in 22.5% of PL cases (n=9 of 40) showing a high PD-L1 score in 77% of cases (n=7 of 9). In one case, both PD-1 and PD-L1 were expressed in tumor cells (Patient #54).
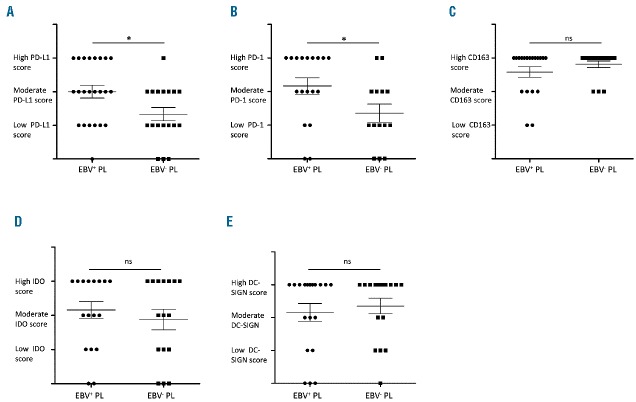
We next compared the distribution and the expression of PD-1, PD-L1, IDO, DC-SIGN and CD163 between EBV+ PL and EBV−PL samples (Figure 2). Mean rates of PD-L1 and PD-1 expression in each group were significantly higher in the microenvironment of EBV+ PL than in EBV−PL (P=0.02 and P=0.03, respectively) (Figure 2A and B). The percentage of PD-L1+ immune cells per sample was nearly 2-fold higher in EBV+ PL than in EBV− PL. In contrast, EBV+PL and EBV−PL samples showed a similar rates of CD163+ cell staining (Figure 2C) and were similar for IDO and DC-SIGN expression in the PL microenvironment (Figure 2D and E). Interestingly, strong expression of PD-L1 in tumor cells was observed in the majority of EBV+PL cases (n=7 of 9) (P=0.01). Moreover, the majority of PL cases with high PD-L1 score in tumor cells were EBV+PL, whereas tumor cells in 2 EBV−PL cases showed only low (n=1) or moderate (n=1) PD-L1 score. Furthermore, the single case co-expressing PD-1 and PD-L1 was an EBV+PL (Patient #54).
Figure 2.
Rates of infiltrating immune cells expressing immune escape markers in Epstein-Barr virus (EBV)+ versus EBV− plasmablastic lymphomas (PL). Immune checkpoint scores of (A) PD-L1, (B) PD-1, (C) CD163, (D) IDO and (E) DC-SIGN stainings in immune cell infiltrates of EBV+ and EBV− PL. *Significant differences between groups (P=0.02, P=0.03, P=0.46, P=0.24, respectively).
Prognostic impact of EBV status in plasmablastic lymphoma patients
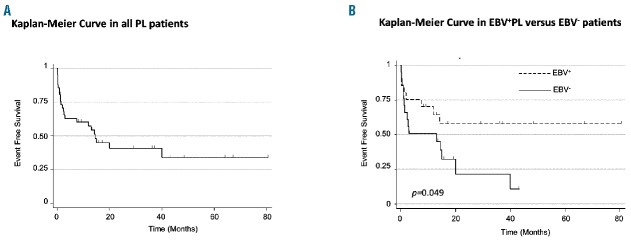
Clinical outcome was available in 47 patients. After a median follow up of 10.5 months (range 1 week-80 months), 51% of patients died, 6% were alive with stable or progressive disease, and 43% were alive and in complete remission. In the whole cohort, 2-year event-free survival was 40.8% (95%CI: 24%–57%) (Figure 3A). The 2-year event-free survival was significantly shorter for EBV−PL patients than for EBV+ PL patients (22% vs. 58%, respectively; P=0.049) (Figure 3B). Multivariate analysis confirmed EBV status as an independent prognostic factor (Online Supplementary Table S3). In contrast, MYC rearrangement status and PD-1/PD-L1 overexpression (with cut off: ICP score ≥3) were not associated with survival (Online Supplementary Table S3).
Figure 3.
Event-free survival in plasmablastic lymphoma (PL) patients. Kaplan-Meier curve showing event-free survival in all (A) PL patients and (B) Epstein-Barr virus (EBV)+ and EBV− PL patients. Event was defined as death or disease progression.
Discussion
We report here the largest known clinico-pathological analysis of PL cases arising in HIV+ and HIV− patients. In accordance with previous reports, most PL occurring in the context of immune deficiency (mainly an HIV infection or immunosuppressive treatment for transplantation) were associated with EBV.5,38–40 Our series shows a lower frequency of EBV+PL than previously reported,5,6,41,42 which may be due, at least in part, to the smaller number of patients with an underlying immune deficiency. No differences in histological and immunophenotypic features were observed between EBV+ and EBV− PL. The distinction between EBV− PL and plasmacytoma could be challenging, but unlike PL, plasmacytoma usually arises in immunocompetent patients and is composed of mature plasma cells without cytological atypia. However, MYC rearrangement was observed significantly more often in EBV+PL than in EBV−PL, in agreement with previous reports.6,41,42 Notably, all cases harboring MYC rearrangement had strong expression of MYC protein, which was also observed in 66% of PL cases without MYC rearrangement. MYC rearrangement has been reported to be the commonest chromosomic alteration in PL and was initially proposed as an aggressive factor in PL behavior.9 However, consistent with other reports,38,42 we found that MYC rearrangement did not impact survival. In addition, all but one PL case did not have BCL6 rearrangement at the major breakpoint region; BCL2 rearrangement was also negative in all cases.9,41
Our study suggests that PL develops several patterns of immune escape by expressing a number of immune checkpoint markers. Indeed, we found that nearly all PL express PD-L1 and PD-1 in the immune infiltrate, and that one-quarter of them strongly express PD-L1 in tumor cells and in immune cells. We also show that the PD-1/PD-L1 axis is more over-expressed in the microenvironment in EBV+PL, which is typically associated with situations of immunodeficiency. These findings suggest that an antiviral response against EBV may favor the recruitment of immune cells PD-L1. In this regard, it has been shown that cytokines, such as interferon γ, can also potentially up-regulate PD-L1 on macrophages via the ISRE/IRF1 motif in the PD-L1 (CD274) promoter, and thus favor PD-L1 expression in immune cell infiltrates during inflammatory responses.10,43,44 Interestingly, all but 2 cases expressing PD-L1 in tumor cells were associated with EBV infection, which could be consistent with the view that PD-L1 expression is promoted by LMP1 of EBV which increases PD-L1 promoter expression.25,43 However, the majority of EBV+PL tumor cells expressing PD-L1 had an EBV latency type 1 and did not express LMP1 (n=7 of 9) (data not shown), suggesting that PD-L1 can be up-regulated by other mechanisms. In this regard, it has been shown that PD-L1 expression can be driven by intrinsic genetic aberrations of 9p24.1 (a genomic region including CD274, PDCD1LG2 and JAK2) and by the dysregulation of JAK/STAT pathway.43,45,46 As suggested for EBV+ CD20+ diffuse large B-cell lymphoma (DLBCL),25,47 further genetic analyses of PD-L1 (CD274) locus are needed to identify the intrinsic mechanism of PD-L1 upregulation by malignant cells in PL.
In this study we found that PL patients (either associated or not with EBV) showed a high content of CD163+ macrophages, which are known to be associated with immunoregulatory function by promoting Th2 immune responses to the detriment of Th1 anti-tumor immune responses. Furthermore, PLs express IDO and DC-SIGN molecules in a very similar manner in the histiocytic/dendritic cell microenvironment. These immune evasion proteins have been known to be involved in negative immunoregulatory response. For instance, IDO attenuates T-cell clonal expansion, and induces anergy and apoptosis on effector T cells. Nevertheless, reported data differ as to the prognostic impact of PD-1/PD-L1 and IDO expression in lymphoma.16,17,48,49 In spite of this, in our cohort, we did not find any correlation between immune checkpoint expression and PL patients’ survival, and further biological studies on larger cohorts are needed to really evaluate the impact of the immune escape checkpoint on PL prognosis. Altogether, given their association with oncogenic viruses and immunodeficiency, PL should provide attractive targets for immune-based therapy.
Finally, this study confirms the prognostic value of EBV status in PL.6 Indeed, EBV+PL had a better event-free survival, which nevertheless contrasts with the aggressive clinical course of EBV+DLBCL patients.50
In conclusion, PL expresses immune checkpoint proteins as PD-1/PD-L1 in both the microenvironment and in the malignant cells, particularly in EBV+PL. Anti-PD-1 monoclonal antibodies recently received FDA approval for advanced melanoma or non-small cell lung carcinoma and promising data on therapeutic response were also seen in Hodgkin lymphoma.20–24 Therefore, our findings constitute a strong rationale for testing anti-PD-1 monoclonal antibodies in the treatment of PL, a severe and often chemoresistant form of lymphoid malignancy.
Acknowledgments
We thank Laurence Jalabert, Audray Benest and Gabrielle Perez for immunohistochemistry staining and FISH studies (IUCT, Toulouse, France) and Marina Bousquet for reviewing the paper. We thank François-Xavier Frenois for whole slide imaging (IUCT Toulouse, France). We thank the Lymphopath consortium for sending their samples: Pr Gaulard (Hôpital Henri Mondor, Créteil, France); Pr J.F. Fléjou (Hôpital Saint Antoine, Paris, France); Pr V. Costes Martineau (CHU de Montpellier, France); Pr A. de Mascarel et Dr M. Parrens (CHU de Bordeaux, France); Dr I. Soubeyran (Institut Bergonié, Bordeaux, France); Dr C Chassagne-Clement (Centre Léon Bérard, Lyon, France); Dr L. Xerri (Institut Paoli Calmettes, Marseille, France); Dr A Moreau (CHU de Nantes, France); Dr F. Arbion (CHU de Tours, France); Dr I. Quintin-Roué (CHU de Brest, France); Dr J.M Picquenot (Centre Henri Becquerel, Rouen France); Pr L. Martin (CHU de Besançon-Dijon, France); Pr J.M. Vignaud and Dr C. Bastien (CHU de Nancy, France); Pr F. Labrousse (CHU de Limoges, France).
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/101/8/976
Funding
This work was supported in part by institutional grants from the Ligue Regionale de Lutte Contre le Cancer (Canceropole Grand Sud Ouest), the Institut National du Cancer (INCA), the Association de Recherche Contre le Cancer (ARC) (subvention PJA 20131200091), the Laboratoire d’Excellence Toulouse Cancer (TOUCAN) (contract ANR11-LABX), the Programme Hospitalo-Universitaire en Cancérologie CAPTOR (contract ANR11-PHUC0001), and the Institut Carnot Lymphome (CALYM). PB is supported by the Institut Universitaire de France.
References
- 1.Delecluse HJ, Anagnostopoulos I, Dallenbach F, et al. Plasmablastic lymphomas of the oral cavity: a new entity associated with the human immunodeficiency virus infection. Blood. 1997;89(4):1413–1420. [PubMed] [Google Scholar]
- 2.Swerdlow SH, Campo E, Harris NL, et al. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues (4th ed). Lyon, France, IARC Press, 2008. [Google Scholar]
- 3.Rafaniello Raviele P, Pruneri G, Maiorano E. Plasmablastic lymphoma: a review. Oral Dis. 2009;15(1):38–45. [DOI] [PubMed] [Google Scholar]
- 4.Colomo L, Loong F, Rives S, et al. Diffuse large B-cell lymphomas with plasmablastic differentiation represent a heterogeneous group of disease entities. Am J Surg Pathol. 2004;28(6):736–747. [DOI] [PubMed] [Google Scholar]
- 5.Morscio J, Dierickx D, Nijs J, et al. Clinicopathologic comparison of plasmablastic lymphoma in HIV-positive, immunocompetent, and posttransplant patients: single-center series of 25 cases and meta-analysis of 277 reported cases. Am J Surg Pathol. 2014;38(7):875–886. [DOI] [PubMed] [Google Scholar]
- 6.Loghavi S, Alayed K, Aladily TN, et al. Stage, age, and EBV status impact outcomes of plasmablastic lymphoma patients: a clinicopathologic analysis of 61 patients. J Hematol Oncol. 2015;8:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carbone A, Gloghini A. Plasmablastic lymphoma: one or more entities¿ Am J Hematol. 2008;83(10):763–764. [DOI] [PubMed] [Google Scholar]
- 8.Covens K, Verbinnen B, Geukens N, et al. Characterization of proposed human B-1 cells reveals pre-plasmablast phenotype. Blood. 2013;121(26):5176–5183. [DOI] [PubMed] [Google Scholar]
- 9.Bogusz AM, Seegmiller AC, Garcia R, Shang P, Ashfaq R, Chen W. Plasmablastic lymphomas with MYC/IgH rearrangement: report of three cases and review of the literature. Am J Clin Pathol. 2009; 132(4):597–605. [DOI] [PubMed] [Google Scholar]
- 10.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andorsky DJ, Yamada RE, Said J, Pinkus GS, Betting DJ, Timmerman JM. Programmed death ligand 1 is expressed by non-hodgkin lymphomas and inhibits the activity of tumor-associated T cells. Clin Cancer Res. 2011;17(13):4232–4244. [DOI] [PubMed] [Google Scholar]
- 12.Laurent C, Charmpi K, Gravelle P, et al. Several immune escape patterns in non-Hodgkin’s lymphomas. Oncoimmunology. 2015;4(8):e1026530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armand P. Checkpoint blockade in lymphoma. Hematology Am Soc Hematol Educ Program. 2015;2015(1):69–73. [DOI] [PubMed] [Google Scholar]
- 14.Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010; 22(2):231–237. [DOI] [PubMed] [Google Scholar]
- 15.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122(3):787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choe JY, Yun JY, Jeon YK, et al. Indoleamine 2,3-dioxygenase (IDO) is frequently expressed in stromal cells of Hodgkin lymphoma and is associated with adverse clinical features: a retrospective cohort study. BMC Cancer. 2014;14:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor MW, Feng GS. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J. 1991;5(11):2516–2522. [PubMed] [Google Scholar]
- 18.Kim R, Emi M, Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology. 2007; 121(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013; 14(10):1014–1022.24048123 [Google Scholar]
- 20.Westin JR, Chu F, Zhang M, et al. Safety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: a single group, open-label, phase 2 trial. Lancet Oncol. 2014;15(1):69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ansell SM. Targeting immune checkpoints in lymphoma. Curr Opin Hematol. 2015; 22(4):337–342. [DOI] [PubMed] [Google Scholar]
- 22.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372(4):311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Novakovic BJ. Checkpoint inhibitors in Hodgkin lymphoma. Eur J Haematol. 2016; 96(4):335–343. [DOI] [PubMed] [Google Scholar]
- 24.Cheah CY, Fowler NH, Neelapu SS. Targeting the programmed death-1/programmed death-ligand 1 axis in lymphoma. Curr Opin Oncol. 2015;27(5):384–391. [DOI] [PubMed] [Google Scholar]
- 25.Chen BJ, Chapuy B, Ouyang J, et al. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res. 2013;19(13):3462–3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Twa DD, Chan FC, Ben-Neriah S, et al. Genomic rearrangements involving programmed death ligands are recurrent in primary mediastinal large B-cell lymphoma. Blood. 2014;123(13):2062–2065. [DOI] [PubMed] [Google Scholar]
- 27.de Leval L, Parrens M, Le Bras F, et al. Angioimmunoblastic T-cell lymphoma is the most common T-cell lymphoma in two distinct French information data sets. Haematologica. 2015;100(9):e361–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laurent C, Delas A, Gaulard P, et al. Breast implant-associated anaplastic large cell lymphoma: two distinct clinicopathological variants with different outcomes. Ann Oncol. 2016;27(2):306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275–282. [DOI] [PubMed] [Google Scholar]
- 30.Green TM, Young KH, Visco C, et al. Immunohistochemical double-hit score is a strong predictor of outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30(28):3460–3467. [DOI] [PubMed] [Google Scholar]
- 31.Meyer PN, Fu K, Greiner TC, et al. Immunohistochemical methods for predicting cell of origin and survival in patients with diffuse large B-cell lymphoma treated with rituximab. J Clin Oncol. 2011;29(2): 200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laurent C, Muller S, Do C, et al. Distribution, function, and prognostic value of cytotoxic T lymphocytes in follicular lymphoma: a 3-D tissue-imaging study. Blood. 2011;118(20):5371–5379. [DOI] [PubMed] [Google Scholar]
- 33.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taube JM, Anders RA, Young GD, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4(127):127ra37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laurent C, Guerin M, Frenois FX, et al. Whole-slide imaging is a robust alternative to traditional fluorescent microscopy for fluorescence in situ hybridization imaging using break-apart DNA probes. Hum Pathol. 2013;44(8):1544–1555. [DOI] [PubMed] [Google Scholar]
- 36.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17(4):1244. [DOI] [PubMed] [Google Scholar]
- 37.Hsi ED, Lorsbach RB, Fend F, Dogan A. Plasmablastic lymphoma and related disorders. Am J Clin Pathol. 2011;136(2):183–194. [DOI] [PubMed] [Google Scholar]
- 38.Castillo JJ, Furman M, Beltran BE, et al. Human immunodeficiency virus-associated plasmablastic lymphoma: poor prognosis in the era of highly active antiretroviral therapy. Cancer. 2012;118(21):5270–5277. [DOI] [PubMed] [Google Scholar]
- 39.Cattaneo C, Re A, Ungari M, et al. Plasmablastic lymphoma among human immunodeficiency virus-positive patients: results of a single center’s experience. Leuk Lymphoma. 2015;56(1):267–269. [DOI] [PubMed] [Google Scholar]
- 40.Guan B, Zhang X, Hu W, et al. Plasmablastic lymphoma of the oral cavity in an HIV-negative patient. Ann Diagn Pathol. 2011;15(6):436–440. [DOI] [PubMed] [Google Scholar]
- 41.Boy SC, van Heerden MB, Babb C, van Heerden WF, Willem P. Dominant genetic aberrations and coexistent EBV infection in HIV-related oral plasmablastic lymphomas. Oral Oncol. 2011;47(9):883–887. [DOI] [PubMed] [Google Scholar]
- 42.Valera A, Balague O, Colomo L, et al. IG/MYC rearrangements are the main cytogenetic alteration in plasmablastic lymphomas. Am J Surg Pathol. 2010;34(11):1686–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Green MR, Monti S, Rodig SJ, et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 2010;116(17):3268–3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5(4):263–274. [DOI] [PubMed] [Google Scholar]
- 45.Green MR, Rodig S, Juszczynski P, et al. Constitutive AP-1 activity and EBV infection induce PD-L1 in Hodgkin lymphomas and posttransplant lymphoproliferative disorders: implications for targeted therapy. Clin Cancer Res. 2012;18(6):1611–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marzec M, Zhang Q, Goradia A, et al. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD-L1, B7-H1). Proc Natl Acad Sci USA. 2008; 105(52): 20852–20857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nicolae A, Pittaluga S, Abdullah S, et al. EBV-positive large B-cell lymphomas in young patients: a nodal lymphoma with evidence for a tolerogenic immune environment. Blood. 2015;126(7):863–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Godin-Ethier J, Hanafi LA, Piccirillo CA, Lapointe R. Indoleamine 2,3-dioxygenase expression in human cancers: clinical and immunologic perspectives. Clin Cancer Res. 2011;17(22):6985–6991. [DOI] [PubMed] [Google Scholar]
- 49.Kiyasu J, Miyoshi H, Hirata A, et al. Expression of programmed cell death ligand 1 is associated with poor overall survival in patients with diffuse large B-cell lymphoma. Blood. 2015;126(19):2193–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oyama T, Yamamoto K, Asano N, et al. Age-related EBV-associated B-cell lymphoproliferative disorders constitute a distinct clinicopathologic group: a study of 96 patients. Clin Cancer Res. 2007;13(17): 5124–5132. [DOI] [PubMed] [Google Scholar]