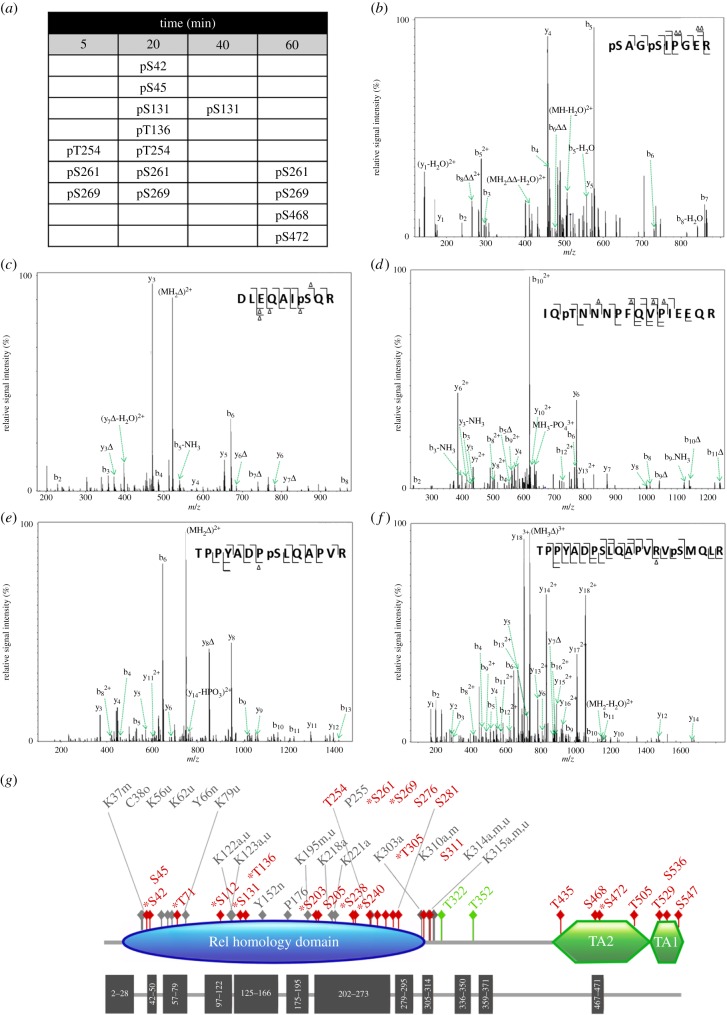
Figure 1.
TNFα induces dynamic multi-site phosphorylation of endogenous RelA. (a) Phosphorylation sites identified at 5, 20, 40, 60 min post-stimulation of SK-N-AS cells with the cytokine TNFα are detailed. No phosphorylation sites were observed in the absence of stimulation. CID product ion spectra of a (b) doubly charged ion at m/z 437.7, indicating phosphorylation of Ser42 and Ser45; (c) doubly charged ion at m/z 570.1, indicating phosphorylation of Ser131; (d) triply charged ion at m/z 669.7, indicating phosphorylation of Ser136; (e) doubly charged ion m/z 796.2, indicating phosphorylation of Ser261; (f) triply charged ion at m/z 769.2, indicating phosphorylation of Ser269. (g) Schematic of RelA detailing known and novel (*) sites of modification. Phosphorylation sites are in red, glycosylation sites in green; a, acetylation; m, methylation; n, nitrosylation; o, oxidation; u, ubiquitination; TA, transactivation domain. Dark grey blocks represent those regions in the primary sequence identified by shotgun LC–MS/MS analysis following proteolytic cleavage with different enzymes.