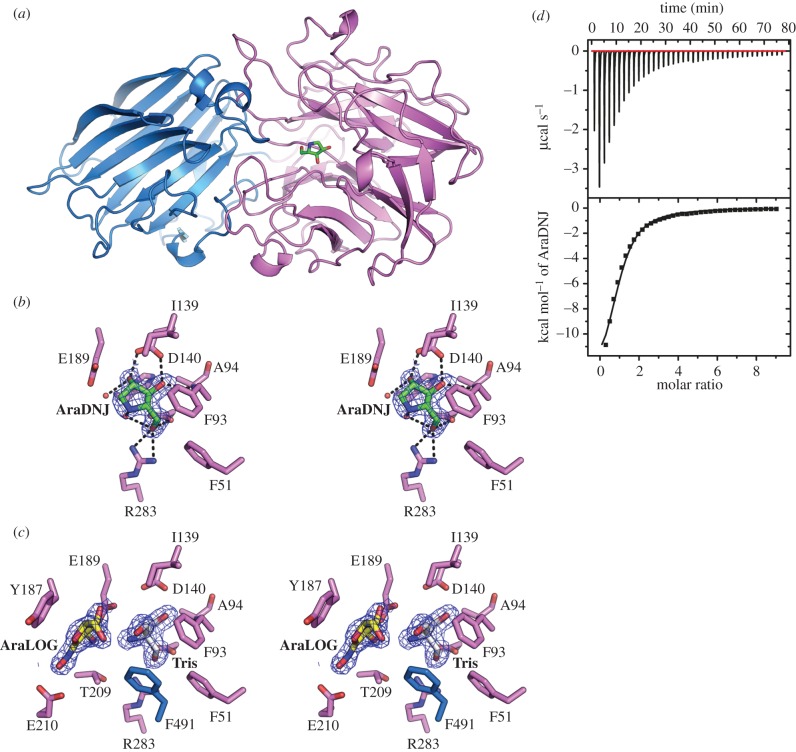
Figure 3.
Overall structure and inhibitor binding to BoGH43A α-l-arabinofuranosidase. (a) Overall structure of BoGH43A; the N-terminal catalytic domain is coloured purple and C-terminal β-sandwich domain is coloured blue. The location of the active site is indicated by the position of AraDNJ shown in stick representation coloured by atom type with green carbons. (b) Wall-eyed stereo view of the active site in the BoGH43A-AraDNJ complex. The final 2Fo-Fc map for the ligand is shown contoured at 1σ in blue. The hydrogen bonding interactions made by the inhibitor are shown as black dashed lines. (c) Wall-eyed stereo view of the active site for BoGH43A-AraLOG complex. Binding of the AraLOG inhibitor (yellow carbon atoms) was too weak to displace TRIS (white carbon atoms) from the −1 sub-site and instead occupies +1, revealing key stacking interactions with Tyr187 and other conserved residues. (d) ITC thermogram showing the binding of AraDNJ to BoGH43A in solution giving a Kd of 35 ± 4 µM.