Figure 4. RING1 opening of E2~Ub enforces transfer via the RING2 active site.
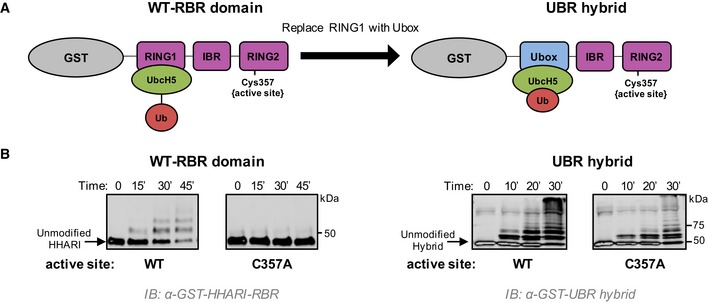
- Schematic representation of the two constructs used. Left: GST‐tagged HHARIRBR with its native RING1 domain that does not induce closed conformations of UbcH5˜Ub. Right: GST‐tagged HHARI construct in which the RING1 domain was replaced with the Ubox domain of E4BU to generate the UBR hybrid that promotes closed conformations of UbcH5˜Ub.
- Left: Auto‐ubiquitination assays were performed with wild‐type HHARIRBR or an active‐site‐dead mutant (C357A‐HHARIRBR). Products were visualized by Western blotting against GST. The zero time point was taken immediately prior to ATP addition; all other times are post‐ATP addition. Right: Identical assays as shown on left were performed with the UBR hybrid. The active‐site‐dead mutant (C357A) retains substantial auto‐ubiquitination activity signifying that UbcH5˜Ub is able to transfer Ub directly onto the GST‐UBR hybrid construct, bypassing the RING2 active site Cys.
