Figure 5. The Ub hydrophobic patch plays a role in Ub transfer from E2~Ub onto RING2 of RBR E3 ligases.
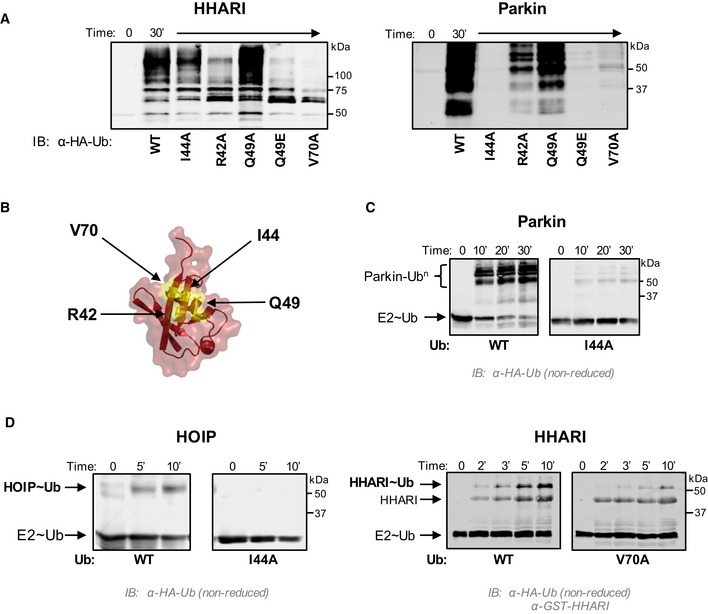
- E3 auto‐ubiquitination assays were performed using various Ub mutants (I44A, R42A, Q49A, Q49E, and V70A) and the RBR E3s HHARIRBR (left) and ParkinRBR (right). Products were visualized by Western blotting against HA‐Ub. Samples were analyzed 30 min after ATP addition.
- The hydrophobic patch of Ub (PDB 1ubq) is colored yellow on a surface representation and positions of each mutation are noted.
- UbcH7˜Ub conjugates were preformed with either WT‐Ub or I44A‐Ub. After the addition of apyrase to quench the charging reaction, UbcH7˜UbWT (left) or UbcH7˜UbI44A (right) was incubated with ParkinRBR. The disappearance of each UbcH7˜Ub species and appearance of auto‐ubiquitinated E3 were visualized under non‐reducing conditions by Western blotting for HA‐Ub. Time was recorded post‐addition of ParkinRBR. E2˜Ub conjugated with I44A‐Ub does not disappear over the time course of the reaction.
- UbcH7 conjugated with WT‐Ub, I44A‐Ub, or V70A Ub as indicated was incubated with either H887A‐HOIPRBR ‐ LDD (left) or H359A‐HHARIRBR (right) mutants that allow trapping of the E3˜Ub thioester with WT‐Ub 29, 41. While a HOIP˜Ub thioester species is observed when UbcH7 was charged with WT‐Ub, no detectable transfer occurs with the I44A‐Ub conjugate (left). Similarly, V70A‐Ub shows reduced formation of the HHARI˜Ub thioester (right). In addition to blotting for HA‐Ub, the blot on the right was also blotted for GST‐HHARI.
