Figure 1.
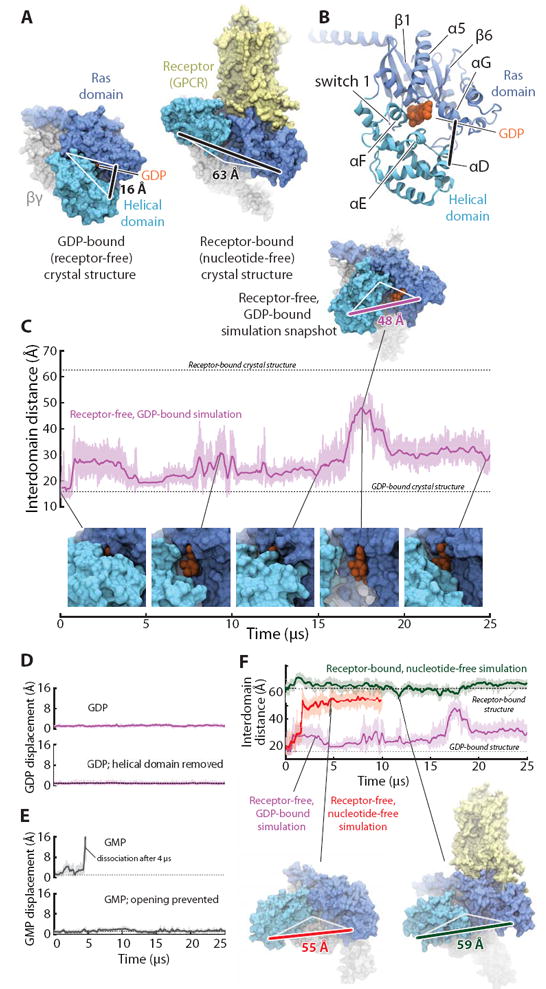
The Ras and helical domains of the G protein α subunit separate spontaneously and frequently when GDP is bound, even in the absence of a receptor. (A) The Ras and helical domains are tightly apposed in all nucleotide-bound G protein crystal structures, enveloping the nucleotide (left: GDP-bound Gt heterotrimer; PDB entry 1GOT), but are dramatically separated in the receptor-bound, nucleotide-free structure (right: β2-adrenergic receptor–Gs heterotrimer [β2AR–Gs] complex; PDB entry 3SN6). GDP is colored orange, the Ras domain blue, the helical domain cyan, Gβγ gray, and the receptor yellow. The degree of domain separation is represented by a thick black line connecting Ala134 and Glu272 in Gαt or the corresponding Ala161 and Glu299 in Gαs, with both ends connected by white lines to a pivot point near Thr166 (Gαt) or Ser193 (Gαs). (B) Key structural motifs of the α subunit, illustrated using the GDP-bound Gt structure. (C) Spontaneous domain separation provides an exit pathway for GDP. In simulations of receptor-free, GDP-bound Gt, the Ala134–Glu272 distance varies substantially as the domains fluctuate between apposed and separated conformations; raw (light purple) and smoothed (250-ns moving average; dark purple) data are shown. Representative molecular simulation snapshots (top: overview; bottom: nucleotide-binding site) display varying degrees of GDP exposure. Data are from simulation 2 (Table S1). (D) Domain separation is not sufficient for rapid nucleotide release. GDP remains tightly bound to receptor-free Gt (top), even with the helical domain removed (bottom; traces show displacement of the centroid of the nucleotide non-hydrogen atoms relative to the crystal structure). Data from simulations 2 and 33. (E) Domain separation is necessary for rapid nucleotide release. GMP dissociates spontaneously from receptor-free Gt (top) but remains bound when the interdomain distance is artificially restrained to prevent domain separation (bottom). Data from simulations 16 and 31. (F) Domain separation is greater in the absence of a nucleotide. In simulations initiated from the receptor-free, GDP-bound Gt crystal structure, but with the GDP removed, the Ras and helical domains exhibited extensive and prolonged separation (red trace; left-hand snapshot). In simulations of the β2AR–Gs complex, also nucleotide-free, the helical domain remained widely separated from the Ras domain, although it typically moved away from the membrane toward the beta propeller of Gβγ (green; right-hand snapshot). GDP-bound Gt simulation data from panel C are replicated for reference (purple). See SM for details on structural renderings. Data from simulations 2, 14, and 22.
